Citronic acid – Wikipedia
| Structural formula | |
|---|---|
 |
|
| General | |
| Name | Citric acid |
| other names |
|
| Mash formula | C 6 H 8 O 7 |
| Brief description |
Colorless, odorless solid [3] |
| External identifiers/databases | |
| Drug | |
| ATC-Code |
A09 Ab04 |
| Characteristics | |
| Molar masses |
|
| Aggregate state |
fest |
| density |
|
| Melting point | |
| boiling point |
Decomposition: from 175 ° C [3] |
| p K S -Value | |
| solubility |
|
| safety instructions | |
| Toxicological data |
3000 mg·kg −1 (LD 50 , Gears, everywhere) [3] |
| As far as possible and common, SI units are used. Unless otherwise noted, the data provided applies to standard conditions. | |
Citric acid [7] (Alternative spelling citric acid [8] ) is a colorless, water -soluble carboxylic acid, which is one of the tricarbo acids and one of the fruit acids. In addition to the water -free variant, the citronic acid sounds (C 6 H 8 O 7 · H 2 O) that contains a molecule of crystal water per molecule. The salts and esters of the citric acid are the citrate. A constitutional isomer of citronic acid is isocitronic acid.
Story
Carl Wilhelm Scheele isolated citric acid from lemon juice for the first time in 1784 [9] – therefore the name. However, citric acid may have already been known to the first alchemist, albeit names, among other things. As early as the 9th century, the Arabic alchemist Dschābir Ibn Hayyān (Geber) is said to have discovered citronic acid.

Happen

Citronic acid is one of the most widespread acids in the plant kingdom and occurs as a metabolic product in all organisms. For example, lemon juice contains 5–7% citric acid. But it also occurs in apples, pears, sour cherries, raspberries, blackberries, currants, in coniferous wood, mushrooms, tobacco leaves, in wine and even in the milk.
Citronic acid is widespread because it (giving the name) as an intermediate in the citratzycle (also tricarboxylic acid cycle, cancer cycle). This process takes on a key role in carbohydrate and fatty acid metabolism of all oxygen-consuming living things, including humans. This cycle also provides the basic structures for the structure of most amino acids.
Extraction and presentation
Production of citrus fruits
According to the original procedure, citric acid was obtained from citrus fruits. [ten] Lemon juice was moved with concentrated ammonia solution, thickened and filtered. The slightly soluble ammonium citrate was converted into a heavier, soluble calcium citrate by fitting with calcium chloride. The solution was filtered again and the filter cake was solved in 25%sulfuric acid, with even poorer -soluble calcium sulfate (plaster). After renewed filtration, a citronic acid solution is obtained. The pure citric acid is obtained from crystallization.
Biotechnical production
Industrially, citric acid is produced by fermentation of sugary raw materials such as molasses and corn. [11] For fermentation Aspergillus-niger -A -stems used. In the USA and China, transgenic variants of mold are often used in the USA and China, this is not permitted in Europe. In the case of citronic acid production, three conditions must be met:
- High glucose and oxygen content in the nutrient medium
- Low pH value (PH <3). On the one hand, this inhibits the consequence of the CitraTatsynthase in the citratzycle, the aconitas. Such a low pH is far from the pH optimum of the enzyme and significantly reduces its activity. As a result, the mushrooms only metabolize the citric acid to a small extent. On the other hand, the outer membrane of the mushroom cells becomes unstable and the citronic acid is released into the outer medium. In addition, the risk of contamination due to undesirable foreign organisms is low in such low pH.
- Low Fe 2+ Concentration (<5 mg/l). As a result, the aconite is missing the cofactor. The fairy 2+ -IONIONS are bound by adding potassium hexacyanidoferrat (III). Mn 2+ -Concentrations (<2 µg/l) also lead to high citrate outlets.
Purity and tolerance of citronic acid on the market show great differences. Various Aspergillus niger strains produce mycotoxins under certain growth conditions. The production of these substances does not take place under controlled conditions. There are no residues of mold in highly cleaned citronic acid.
Characteristics


Physical Properties
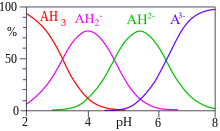
Citric acid forms rhombic crystals in anhydrous condition that taste slightly acidic. An aqueous solution to the citronic acid leads the electrical current, since the carboxy groups split protons and thus existing load carriers (ions) are available in the solution. The acid editing constants of the citric acid are PK s first = 3,13, pK s 2 = 4.76 and PK s 3 = 6.4. The acid nest in the citronic acid, partly or completely dissociated, is called a citrate. For solubility in water, the values are very different in the literature. [6] [twelfth] [13]
Chemical properties
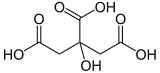
Due to their three carboxy groups (–cooh), citric acid is one of the organic tricarboxylic acids. In addition, the hydroxy group (–OH) in position 3 of the carbon backbone shows it as hydroxy carbonic acid. Citronic acid is a chelator for metal ions.
Citronic acid is received by many reactions typical of carboxylic acids:
Citronic acid can be oxidized with different oxidizing agents (e.g. peroxides or hypochlorites). Depending on the reaction conditions, β-ketoglutaric acid, oxalic acid, carbon dioxide and water can arise.
In small quantities, citric acid indirectly promotes bone growth because it favors the absorption of calcium. In larger quantities, however, it works toxic (LD 50 With rats: 3 g/kg). [3]
Read
The hydroxy group (–OH) is located in position 2 of the carbon backbone in isocitronic acid. It differs from the citric acid in the position of this hydroxy group, which is 3rd in the citric acid.
use

Citronic acid is often contained in limestone cleaning agents because it is odorless. Due to the acidic effect, it solves calcium carbonate to the water-soluble calcium dicitrato complex, a typical chelat complex [ca (cit) 2 ] 4− . The complex disintegrates due to increasing temperature and increasing pH, water-insoluble calcium citrate approx. 3 (Cit) 2 out of. In the event of an excess of citronic acid, calcium citrate dissolves again. [14] Due to the risk of constipation, it is not recommended to use citronic acid solution as descaler for heated pump-powered pipeline systems such as coffee machines or heat exchangers.
To solve lime and iron and manganese compounds, which form when the fountain is discharged, citronic acid is used together with a low addition of ascorbic acid as a reduction agent (alternatively sodium dithionit). [15]
Citronic acid and their salts are used for preservation and also for homogenization as a melting salt as well as as an acid remedy or acid regulator of food, for example in drinks. It is included in shower powder and stick together with sodium hydrogen carbonate. As a natural part of most fruits, it is contained in fruit juices (fruit acids). Citronic acid is in the EU as a food additive under the number And 330 approved in most foods. An exception is chocolate products and fruit juices, for which there is only limited approval, as well as some foods, such as honey, milk and butter, for which there is no approval. [16] Since it acts as a peptisator, it is used to produce stable suspensions. In fish dishes, she can convert biogenic amine into non -fleeting salts and thus reduce the fish smell.
The multiple use of citric acid in food is criticized because it solves aluminum as a complex educator and increases its absorption. High content of citronic acid favors the demineralization of tooth enamel, which can lead to dental or caries. [17] In the case of so-called “Candy sprays”-confectionery offered in pump sprays and sprayed directly into the mouth-there is also a risk that mucous membranes will be irritated due to the high citronic acid content or there are breathing problems. [18]
Citric acid and citrate prevent blood clotting. Therefore, donations are preserved in bags that contain a citrate buffer solution. Citrate blood is used for coagulation analyzes, in which blood is mixed with citrate in a ratio of 9: 1 (9 parts blood + 1 part 0.11 mol/l sodium citrate). A special use is the use in cell separators. A vein is removed from a vein, the desired blood components (e.g. thrombocytes) are separated in the device, and the remaining blood is attributed to the vein. So that the blood in the device does not form a dangerous clot, it is moved with citrate buffer.
Citronic acid, like EDTA, is used as a fluff solution for root canal treatments in dentistry.
Other areas of application of citronic acid:
- Citronic acid is used as water -hardening and alternative fabric softeners.
- Citronic acid is used to passivate stainless steel. In this process, the free iron shares are solved from the surface. This affects the chrome iron ratio, which leads to an improvement in the passive layer and thus to improve corrosion protection.
- Citronic acid becomes the pH value adjustment of cosmetics, e.g. B. skin care notion or cream used. [19] [20]
- In high doses, citric acid also serves as a rust remover.
- Citronic acid is used as a catalytic converter in organic hard work by hydrothermal carbonization. [21]
Citronic acid trimethyl ester, citric acid acid hylester and citronic acid n -Butylester are used as plasticizers, among other things.
literature
Weblinks
Individually
- ↑ Entry to CITRIC ACID in the Cosing database of the EU Commission, accessed December 28, 2019.
- ↑ Entry to E 330: Citric acid in the European database for food additives, accessed on June 27, 2020.
- ↑ a b c d It is f g Entry to citric acid In the Gestis fabric database of the IFA, accessed on January 3, 2023. (JavaScript required)
- ↑ a b c d Entry to Citric acid . In: Römpp Online. Georg Thieme Verlag, accessed on July 11, 2014.
- ↑ a b c Christian Beyer: Quantitative inorganic analysis: a companion for theory and practice . Springer Science & Business, 1996, ISBN 3-528-06779-9, S. 96 ( limited preview in the Google book search).
- ↑ a b c d It is data sheet Citric acid at Sigma-Aldrich, accessed on November 30, 2009 ( PDF ).
- ↑ Karl-Friedrich Arndt, Axel Satzger: Langenscheidt specialist dictionary chemistry and chemical technology English German-English . Ed.: Technical University of Dresden. Langenscheidt, Berlin 2009, ISBN 978-3-86117-476-9 ( Online specialist dictionaries from Langenscheid Chemistry: Search for citronic acid and citric acid ).
- ↑ Dictionary of the Council for German Spelling
- ↑ Carl Wilhelm Scheele: Note about lemon juice, as well as ways to crystallize the same . In: The new actions of the Royal Science Academy . Band 5 . Stockholm 1784, S. 105–109 ( biodiversitylibrary.org ).
- ↑ Frank H. Verhoff: Citric Acid. In: Ullmann’s Encyclopedia of Industrial Chemistry , 2005, Wiley-VCH, Weinheim.
- ↑ Walid A. Lotfy, Khalid M. Ganem, Ehab R. El-Halaow: Citric acid production by a novel Aspergillus niger isolate: II. Optimization of process parameters through statistical experimental designs. In: Bioresource Technology (2007), Band 98, Heft 18, S. 3470–3477, doi:10.1016/j.biortech.2006.11.032 . PMID 17317159 .
- ↑ data sheet Citric acid at Merck, accessed on March 5, 2016.
- ↑ OECD: Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Citric acid , accessed on March 5, 2016.
- ↑ Martina Vavrusova, Leif H. Skibsted: Aqueous solubility of calcium citrate and interconversion between the tetrahydrate and the hexahydrate as a balance between endothermic dissolution and exothermic complex formation . In: International Dairy Journal . Band 57 , June 2016, S. 20–28 , doi: 10.1016/j.idairyj.2016.02.033 : „Excess citrate increased calcium citrate solubility but decreased the calcium ion activity of the saturated solution […]“
- ↑ Patent Nr.: DE19953807A1; Patent Nr.: EP 0456271A1.
- ↑ Zzulv: Appendix 4 (to Section 5 (1) and Section 7) Limited added substances ( Memento from February 28, 2018 in Internet Archive ).
- ↑ High content of citric acid in confectionery and drinks increase the risk of tooth damage. (PDF) In: Federal Institute for Risk Assessment. January 9, 2004, accessed on July 20, 2022 .
- ↑ Evaluation of “Candy Sprays” with an increased lemon acid content. (PDF) In: BfR. January 21, 2013, accessed on July 20, 2022 .
- ↑ Vollrath, jump: Citronic acid: Example of a biotechnical product. In: Basics of the Life Sciences , Wiley-VCH Verlag, Weinheim 2000, ISBN 978-3-527-29560-9.
- ↑ Professor Blume’s media offer: Citronic acid in cosmetics .
- ↑ Peter Brandt: The “hydrothermal carbonization”: a remarkable way to develop CO 2 to minimize or even avoid? In: Journal for consumer protection and food safety , 2009, 4 (2), S. 151–154; two: 10.1007/s00003-009-0472-7 .

Recent Comments