Food – Wikipedia
| Structural formula | |
|---|---|
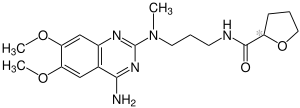 |
|
| Simplified structural formula without stereochemistry | |
| General | |
| Freiname | Alfuzosin |
| other names |
|
| Mash formula | |
| External identifiers/databases | |
| Drug | |
| ATC-Code |
G04 CA01 |
| Active ingredient class |
Alphablocker |
| Mechanism |
a first -Adrenoreceptor antagonist |
| Characteristics | |
| Molar masses |
|
| Aggregate state |
fest |
| Melting point |
225 ° C (hydrochloride) [first] |
| p K S -Value |
8.13 [first] |
| safety instructions | |
| Toxicological data | |
| As far as possible and common, SI units are used. Unless otherwise noted, the data provided applies to standard conditions. | |
Alfuzosin Is a drug from the group of α first -Adrenoreceptor antagonist (alphablocker), which is used in the treatment of a benign prostate hyperplasia (BPH). The chiral connection is derived from the Chinese. It was patented in 1979 and 1982 as an antihypertonic by Synthélabo (today Sanofi). [first]
application areas [ Edit | Edit the source text ]
The drug is used to treat the symptoms of benign prostate hyperplasia. It has no influence on the size of the prostate.
Contraindications (contraindications) [ Edit | Edit the source text ]
In the presence of severe liver sufficiency or orthostatic hypotension (low blood pressure), the administration is contraindicated, as well as treatment with other alpha blockers. [3]
Unwanted effects (side effects) [ Edit | Edit the source text ]
Possible side effects include fatigue, dizziness, headache, flu symptoms and hypotone dysregulation. [3]
As an antagonist, alfuzosin selectively binds to postsynaptic α first -Drenoreceptors and thus relaxes the smooth muscles of prostate and urethra. This increases urinary flow and makes micturition easier. The bioavailability is 64% and the plasmhale dumpling time is 4 to 6 hours. The maximum plasma concentration is reached after 90 minutes. [3]
Alfuzosin contains a stereo center and is therefore a chiral. There are two enantiomeric shapes ( R )-form and ( S )-Shape. However, only the racemat [(( RS ) -Alfuzosin], i.e. a 1: 1 mixture from the ( R ) -Enantiomer and the ( S )-Enantiomer: [4]
| Enantiomeres from Alfuzosin | |
|---|---|
 ( R )-Alfuzosin |
 ( S )-Alfuzosin |
Alfunar (d), [5] Fuzocim (CH), Urion (D), UroXatral (D), Xatral (CH)
- ↑ a b c Entry to Alfuzosin . In: Römpp Online. Georg Thieme Verlag, accessed on September 9, 2014.
- ↑ a b c d data sheet Alfuzosin hydrochloride at Sigma-Aldrich, accessed on May 31, 2022 ( PDF ).
- ↑ a b c Michael C. Truß, Christian G. Stief, Stefan Machtens, Till Wagner, Udo Jonas: Pharmacotherapy in urology. 2nd, completely revised edition. Springer, Heidelberg 2005, ISBN 3-540-23449-7, p. 302 ff.
- ↑ Red List Service GmbH (ed.): Red List 2017 – Drugs for Germany (including EU registrations and certain medical devices) . Rote List Service GmbH, Frankfurt/Main, 2017, ed. 57, p. 159, ISBN 978-3-946057-10-9.
- ↑ Red List 2017, Verlag Rote List Service GmbH, Frankfurt am Main, ISBN 978-3-946057-10-9, p. 159.
Recent Comments