Lilials – SpeedyLook encyclopedia
Liliales It is a taxon taxon belonging to the order taxonomic category, used in modern classification systems such as the APG III classification system of 2009 [ first ] And the APWEB, [ 4 ] And is forcedly circumscribed at least by the Liliaceae family. Liliales is a longly recognized order, but not necessarily delimited as in the classification systems mentioned. Liliales was traditionally a difficult order to circumscribe and divide in families, since their characters do not occur in patterns that delimit groups clearly, and previously it was located within a very wide lilia to many monocotyledons with colorful and without starch in the endosperm in the endosperm , that today are distributed in lilials, godcoreales and asparagrales. The current constituency is endorsed by DNA molecular analyzes that indicate that the order as defined here is monophytic. It is located within the LiliopSida class (= monocotyledonous). The order as currently circumscribed includes 10 families.
The representative members of this order are mainly herbaceous, although there are also lianas and shrubs with organs that store food, such as cormos or rhizomes, and even herbs without chlorophyll and mycheterotrophic (Corsiaceae).
Description [ To edit ]
- Theoretical introduction in descriptive terminology of plants
The group consists mainly of geophytes that carry elliptical leaves with the finely venation. The synapomorphies that support this group include the nectaries mainly at the base of the Tepalos or the filaments, the septal nectaries that almost always are missing, the extrots anteras (which open out of the flower) and the frequent presence of points in the Tepalos, which are usually great. The external epidermis of the seed tegument has a cellular structure and has no phytomelanins (a black crust), the internal part of the seed tegument (tegmen) also has a cellular structure, the two are plesiomorphic (see also Stevenson et al. 2000, [ 5 ] Rudall et al. 2000a [ 6 ] ).
The group includes some species with the largest genomes among angiosperms plants et al. 2003b, [ 7 ] en algunas Melanthiaceae, Liliaceae y Alstroemeriaceae ver Leitch et al . 2005 [ 8 ] ).
In addition, the plants of this order have fructans in the stem, chelidonic acid, have saponins, in some species there are Velan, in others there are cuticular waxes such as parallel platelets, the base of the leaves is not watering, the inflorescence is terminal, there are many Ovules tenuineted by carpel, present coca nuclar (“nucellar cap”), the long style, the stigma capted, the deciduous perianto, the endosperm with cells with thick heicellulosic walls, and without the presence of the mitochondrial gene sdh 3.
Melanthiaceae, Liliaceae, Colchicaceae, al igual que Corsiaceae (Rudall y Eastman 2002 [ 9 ] ) They have tepalos with three leaf traces (that is, they present three vascular beams connected to the central vascular system of the stem). Smilacaceae, on the other hand, has Tepalos with a single foliar trace.
The numerous species that make up this order are distributed throughout the world.
Many lilliales usually have typecorrizas of type Paris (F. A. Smith y Smith 1997 [ ten ] ).
The taxonomic diversity of monocotyledons is presented in detail by Kubitzki (1998, [ 11 ] 2006 [ twelfth ] ).
Next, the list of lilial diversity. The descriptions are deliberately incomplete. For more information follow the links.
Campinematon [ To edit ]The campinematáceas, from Nueva Caledonia and Australia, have the base of the persistent fibrous leaves, the leaves without the fine reticulated venation, of shelter base, the androceo adnate to the base of the Tepalos, the elongated, persistent periato, the angular seeds with tiny embryo. Of the two genres that make up the family, Campynemanthe It has a subumbellada inflorescence, the ovary a little super, and the apex of the fold leaf; Campynema It has extral anteras, multinucleated rug cells, and the ovary ínfero. |
 Melanthiáceas [ To edit ]The melanthiaceas, distributed in temperate regions of the northern hemisphere, especially in East Asia and eastern North America (with connections to Peru), consist of 16 genres from which 5 morphologically distinctive groups can be distinguished, among which are genres as Trillium (previously in Trilliaceae). They have allege -veiled, shelf base leaves, cluster inflorescence, 3 or 4 tepalos, separate styles and dry stigma, the persistent perianto in the fruit, the small embryo. |
Petermanniáceas [ To edit ]The Petermanniáceas, with their only species Petermannia surgeon , are distributed in the central part of the East Coast of Australia, where they are rare. They are rhizomatous woody vines with petiolate leaves with reluctant venation and tsenfalls opposed to the leaves. The small flowers, which are born in cymous inflorescences, have an ovary and the fruit is fleshy. The inflorescence and the tendrils are terminal, but they become opposite to the leaves as they are evicted by the strong growth of an axillary outbreak. |
 Colchicáceas [ To edit ]Colchicaceae, of temperate to tropical regions but absent in South America, are geophytes with cormos or rhizomes that can be recognized by their quite long flowers with six tepalos usually free, each more or less wrapping a yarn, and with nectaries on the surface from above or to the base of the Tepalos, and a super ovary. They are divided into 6 tribes. |
Ripogonás [ To edit ]The ripogonaceas, with their only genre Rhipogonum , are distributed in eastern Australia and New Zealand to New Guinea. Ripogonáceas are climbing that often have thorny stems, but without tendrils. The leaves are often opposite, petiolate, and with a sheet that has 3 strong longitudinal veins and reticulated fine venation. The flowers are rather small and indistinct, the fruit is a berry. |
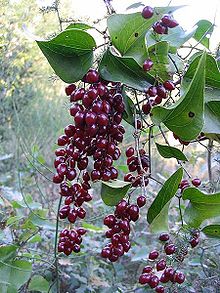 Smilacaceae [ To edit ]The smilacaceae, distributed in paentropical to temperate regions, are often spiny climbing. The leaves are spiral and petiolate, have peciolar even taps, and the sheet has many longitudinal strong veins and reticulated fine venation. The flowers are quite small, typical of monocotyledonous, in umblated inflorescences, the fruit is a berry. |
History of the constituency of order [ To edit ]
Many of the families currently treated within Dioscoreales, Asparagales, and Liliales were previously considered within a broader lilial (Cronchist 1981, [ 13 ] Thorne 1992 [ 14 ] ), Such as the Petaloidal monocotyledons, a “group” characterized by flowers with colorful and without starch in the endosperm. CRONQUIST (1981 [ 13 ] ) He located most of the Petaloidal monocotyledons with 6 stamens flowers in a very wide (and now we know that polyphiletic) Liliaceae. Others have divided the Petaloideas monocotyledons with 6 stamens in Liliaceae, including species with a super ovary, and amaryllidaceae, including species with an ovary ínfero (Lawrence 1951 [ 15 ] ). This separation is also artificial, separating clearly related genres such as Agave and Yucca (Agavaceae) y Crinum (Amaryllidaceae) y Garlic (Alliaceae), as discussed in the treatments of each family.
The concept of lilliales as a distinctive order and separate from other lilioid taxa such as those located in Asparagales and Dioscoreales, originated with Huber (1969, [ 16 ] 1977 [ 17 ] ), Whose ideas were later adopted by Dahlgren et al. (1985 [ 18 ] ). Almost all taxa located in the order were considered closely related, perhaps even members of a single family, Liliaceae. CRONQUIST (1981 [ 13 ] ) He placed all these families along with others such as Veloziaceae and Poncederiaceae in a Liliidae subclass, but if they were not arborescent or did not have reticulated leaves then located them directly in Liliaceae. The reason why such a broad concepts of Liliaceae and Liliidae were formulated is that character patterns did not indicate subgroups clearly delimited, and the authors who segregated some genres in other families did not do it consistently. For example, Cronquist (1981 [ 13 ] ) Segumed Aloe And some of its related “Alfeae”, largely because Aloe He had some arborescent species and the other genera were strongly succulent (for example Haworthia and related), but retained highly related Bulbine in Liliaceae because it was strictly herbaceous. However, Knoophofia , which is herbácea and is not succulent, located it in Alfeae because its flowers and inflorescences were “aloeoids.” Dahlgren et al. (1985 [ 18 ] ) Left a Bulbine and Knoophofia In Asphodelaceae due to their almost identical and strongly bimodal affection, while they placed the others in Alfeae (and the two families in Asparagales due to their seeds with phytomelanins).
Both the Lilial Order and the Liliaceae family are closely delimited, following Dahlgren et al. (1985 [ 18 ] ) And recent cladistic analysis. Huber (1977 [ 17 ] ) Y Dahlgren et al. (1985 [ 18 ] ) Included in the order Iridaceae and Orchidaceae, while in the molecular analysis of DNA these two families appear as part of Asparagales. They also excluded Melanthiaceae and Campynemataceae (such as Melanthiales), which in DNA analyzes appear consistently as members of lilials.
DNA analysis about the sequence rbc L of Chase et al. (1993 [ 19 ] ) And Duvall et al. (1993 [ 20 ] ) They identified a lilial clado containing Alstroemeriae, Colchicaceae, Liliaceae, Melanthiaceae, and Smilacaceae. Chase et al. (1995a [ 21 ] (a) Aggarion Luzuriagaceae, Philesiaceae, Smilacaceae, and Rhipogonaceae, and also ubicaron in order to geeres that it is prevailed habían Sido ubied in the Families Calotae (Hoy en Liliceae (from Liliceae), Trilceae (from Liliceae). (HO IN KOLY IN SE KRICACEAE or Liliaceae) .
In the cladistic analysis made about morphology (not DNA) by Chase et al. (1995b [ 22 ] ) And Stevenson and loconte (1995 [ 23 ] ), Alstroemeriaee, Colchicaceae, Liliaceae, and Melanthiaceae formed a clado, but the other families were further away from this clado. Iridaceae (today in Asparagales) was also included in lilials in the two morphological studies.
The knowledge of relationships within lilia has improved a lot, but the delimitation of some families is still problematic.
- Theoretical introduction in phylogeny
Liliales ( Sense APG 2 2003) Ahora parece ocupar la posición de Clado Hermano de (asparagales + Commelinidae).
Lilial monophilia is supported by cladistic analysis based on morphology and different DNA sequences (Chase et al. 1995a, [ 21 ] b, [ 22 ] 2000, [ 24 ] Davis et al. 2004, [ 25 ] Fay et al. 2006, [ 26 ] Goldblatt 1995, [ 27 ] Graham et al. 2006, [ 28 ] Huhu et al. 2003, [ 29 ] Källersjö et al. 1998, [ 30 ] Solted et al. 2000, [ thirty first ] Stevenson y Loconte 1995, [ 23 ] Winnersen Y Bremer 2001, [ 32 ] See also Chen et al. 2007 [ 33 ] For a Bayesian analysis, but the support of many branches is weak).
The putative phylogenetic relationships within the order are the following:
Colchicaceae, Melanthiaceae and Smilacaceae were traditionally located in Liliaceae.
The complete cladogram is then provided (APWEB, [ 4 ] Updated to July 2008, based mainly on Fay’s analysis et al. 2006, [ 26 ] The relations suggested by the study of rbc L of Janssen y Bremer 2004 [ 34 ] They are quite different, but did not include Petermanniaceae or Corsiaceae):
Alstroeriae (3 Generos, 165 species, Tropical America) is a tropical America of Luzuriagaceae (2 generos, 5 species), native to South America. Luzuriaga ) and Australia and New Zealand ( Drymophila ). The two families share vegetative characters such as being vines with resupinated leaves so that the upper surface during development becomes inferior during maturity, although the ovary is supero in alstroemeriaceae. The two may be combined in a single family (but the APWEB [ 4 ] Even, as of July 2008, it keeps them separate).
Sister of (alstroemeriaceae + lighturiagaceae) is colchicaceae, which is mainly native to the old world, being the only exception THEVULY (of temperate regions of the northern hemisphere). Some genres of Colchicaceae have twisted leaves (as well as alstroemeriae and Luzuriagaceae). Colchicine (an alkaloid used to inhibit the formation of acromatic spindle and cause chromosomal non -disjunction during mitosis, generating a polyploid offspring) is found in all family members.
Petermannia It was included in Colchicaceae in APG (1998 [ 35 ] ) y APG II (2003 [ 2 ] ), But now it is known that the DNA used to do the analysis actually came from a plant of Triplodeia cunninghamii that had been poorly identified as Petermannia (M. W. Chase, unpublished data, cited in Lastis et al. 2005: p.104). The truly Petermannia It turned out to be the sister of the 3 families already mentioned (Colchicaceae, Alstroemeriae and Luzuriagaceae), so it can be appropriate to reinstate the Petermanniaceae family (as suggest et al. 2005 and in fact the APWEB [ 4 ] ).
Melanthiaceae (16 genres, 170 species) was studied in detail by Zomlefer et al. (2001 [ 36 ] ), The family now includes members of the previously known as Trilliaceae. All genres have a distribution in temperate regions of the northern hemisphere, except for a single genre, Schoenocaulon , which is present in Peru, but may have been taken there by humans (they are medicinal plants). The family contains powerful alkaloids. Xerophyllum (two species in North America) is the brother of the genres of the previous Trilliaceae (Chase et al. 1995a, [ 21 ] 1995b, [ 22 ] Rudall et al. 2000a [ 6 ] ). These species are adapted to xerophytic environments (they have thin and graminiform leaves, and habit in a tight rosette), which contrasts with the habit of Trillium and related, which are adapted to living under the canopy of forests and have reticulated leaves. Some authors (Thorne 1992 [ 14 ] ) Considered to FALSE (Melanthiaceae) as one of the most primitive monocotyledons due to its plotted reticulated leaves, and their carpels mostly without merge, but is deeply embedded within lilia and is not at all related to the base of the monocotyledons, which means that it is unlikely you have retained these characters as primarily primitive.
Liliaceae Sense APG is composed of much less genres than in most previous constituencies (for example CRONQUIST 1981 [ 13 ] ). However, as circumscribed by the APG, it is broader than that of others that would have limited the family to only the genres of the nucleus related to Lily (Tamura 1998 [ 37 ] ), That excludes the Calochortus, Prosartes, Tricyrtis , and several more, placing them in Calochortaceae or Trichrideae. Liliaceae is exclusive to temperate regions of the northern hemisphere and is composed of geophytes with many times large and dotted flowers, anteras of extralsa dehiscence, and a super ovary.
Related to Liliaceae are Smilacaceae (Monogenérica, 315 species), almost cosmopolitan, Philesiaceae (2 monoespecific genera), southern South America, and Rhyipogonaceae (monogenérica, 6 species), of Australasia. No characters be known from the DNA that join all these families. Smilacaceae, Philesiaceae, and Rhyipogonaceae have a single spiny pollen (Rudall et al. 2000a [ 6 ] ), But they never formed a clado in molecular analysis. Rhipogonum It is many times located as the brother of Philepea / Lapageria , so it could be combined with them, and Smilax It is generally brother of Liliaceae (but never with more than 80% support). Fay et al. (2006: [ 26 ] Under support), Givnish et al. (2006: [ 38 ] High support), and Chase et al. (2006 [ 39 ] ) They found Philesiaceae and Rhyipogonaceae as brothers taxa, and Smilacaceae as Liliaceae’s brother.
Neyland (2002 [ 40 ] ), Analyzing the variation in the DNA 26S, suggested that Corsiaceae (3 genera, 30 species, China, South America and Australasia) be associated with the lilials. While this position has only weak molecular support, it is long consistent with molecular evidence; Davis et al. (2004 [ 25 ] ) They also found Corsiaceae associated with this order.
Melanthiaceae, Campynemataceae (2 genera, 4 species, Australasia) and Corsiaceae have an unclear pattern of relations with other members of lilials. Campynemataceae was previously considered as related to Melanthiaceae (Dahlgren et al. 1985 [ 18 ] ) Due to its carpels mostly without merge, while Corsiaceae was considered related to Burmanniaceae for its shared mycoparasitic history. However, the two groups of characters are unreliable: free carpels are potentially a simpleiomorphy, while characters associated with mycheterotrophy is convergent even between eudicotyledonous and monocotyledons with the same life story. Campynemataceae and Corsiaceae generally conform to the character pattern observed among the lilial families.
- Theoretical introduction in taxonomy
The order was recognized by APG III (2009 [ first ] ), el Linear APG III (2009 [ 3 ] ) He assigned family numbers 52 to 61. The order had already been recognized by APG II (2003 [ 2 ] ).
Liliales includes 10 families and about 1300 species, the largest families are alstroemeriae, Liliaceae, Colchicaceae, Smilacaceae, and Melanthiaceae.
The complete list of families, according to Angiosperm Phylogeny Website (as of January 2011 [ 4 ] ) That coincides with that of APG III, [ first ] The family numbers assigned according to LAPG III (2009 [ 3 ] ):
The APG II classification system (2003 [ 2 ] ) Used this constituency:
- Alstroemeriaceae
- Campynemataceae
- Colchicaceae
- Corsiaceae
- Liliaceae
- Luzuriagaaceae
- Melanthiaceae
- Pilespeakeae
- Rhipogonaceae
- Smilacaceae
Petermannia It was included in Colchicaceae, today it is known that it was due to an error, so the APWEB and the APG III give it a separate family. In addition, the APG III Anida Luzuriagaceae within Alstroemeriaceae.
In the APG classification system (1998 [ 35 ] ), The constituency was as follows:
- Alstroemeriaceae
- Campynemataceae
- Colchicaceae
- Liliaceae
- Luzuriagaaceae
- Melanthiaceae
- Pilespeakeae
- Ripogonaceae [sic]
- Smilacaceae
The Corsiaceae family was missing, which was later determined that it was a basal lilia.
The Cronquist system (1981 [ 13 ] ) Placed the order in the Liliidae subclass in the liliopsida class [= monocotyledonous] of the Magnoliophyta division [= angiosperms]. The constituency was much broader (many of these plants are now located in Asparagrales and Dioscoreales):
The Thorne System (1992) [ 14 ] He placed the order in the Lilianae superord in the Liliidae Subclass [= monocotyledonous] of the magnoliophasid class [= angiosperms] and used this constituency:
- Alstroemeriaceae
- Campynemataceae
- Colchicaceae
- Iridaceae
- Liliaceae
- Melanthiaceae
- Trilliaceae
The Dahlgren system (1985 [ 18 ] ) Placed the order in the Lilianae superord in the Liliidae subclass [= monocotyledons] of the magnoliopsychosid class [= angiosperms] and used this constituency:
In the Engler classification system (last update in 1964) a similar order was called Lilflorae , located in the Case Monocotyledoneae of the Angiospermae subdivision. Today it is prohibited by the International Botanical Nomenclature Code to use that name for an order (because its suffix does not comply with the rules), but it could be used for a superorder.
The Wettstein system, with its last review in 1935, used names similar to those of the Engler system: the order was called Liliiflorae and located in the Monocotyledones class of the Angiospermae subdivision. In this constituency the order was similar to that of Cronquist.
Another name that was previously used for order was Coronarieae in the Bentham and Hooker system.
Synonyms: Alstroemeriales Hutchinson, Campynematales Doweld, Colchicales Dumortier, Liriales K. Koch, Melanthiales Reveal, Paridales Dumortier, Smilacales Lindley, VERATRALS DUMORTIER – Lilianae Takhtajan, Melanthianae Doweld – Liliidae J. H. Schnaffner – Liliopsida Batsch, In the liriiop Brongniart.
The Lilial Troncal Group is dated in about 124 million years to the present, the Corona Liliales group in about 117 million years to the present (Janssen and Bremer 2004 [ 34 ] ), These estimates are quite different from those of Bremer (2000 [ 41 ] ) That he considered them younger.
See also [ To edit ]
References [ To edit ]
- ↑ a b c d The Angiosperm Phylogeny Group III (“APG III”, en Alfabétic: Brigitta Bremer, Kåre Bremer, Mark W. Chase, Michael F. Fay, James L. Reveal, Douglas E. Soltis, Pamela S. Soltis y Peter F.vens , Además Colaboraron Arne A. Everberg, Michael J. Moore, Richard G. Olmstead, Paula J. Rudall, Kenneth J. Sytsma, David C. Tank, Kenneth Wurdack, Jenny Q.-Y. Xiang y Sue Zmarzty) (2009) . «An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III.» (pdf) . Botanical Journal of the Linnean Society (161): 105-121. Filed from the original May 25, 2017.
- ↑ a b c d APG II (2003). «An Update of the Angiosperm Phylogeny Group Classification for the orders and families of flowering plants: APG II.» (pdf) . Botanical Journal of the Linnean Society (141): 399-436 . Retrieved on January 12, 2009 . (broken link available on the Internet Archive; see the record , the first version and the last ).
- ↑ a b c Elspeth Haston, James E. Richardson, Peter F. Stevens, Mark W. Chase, David J. Harris. The Linear Angiosperm Phylogeny Group (LAPG) III: a linear sequence of the families in APG III Botanical Journal of the Linnean Society, Vol. 161, No. 2. (2009), pp. 128-131. doi:10.1111/j.1095-8339.2009.01000.x Key: citeulike:6006207 pdf: http://onlinelibrary.wey.com/doi/10.1111/j.1095-8339.2009.01000.x/pdf
- ↑ a b c d It is f Stevens, P. F. (2001 en adelante). «Angiosperm Phylogeny Website (version 9, June 2008, and updated since then)» (in English) . Retrieved on July 7, 2008 .
- ↑ Stevenson, D. W.; Davis, J. I., Freudenstein, J. V., Hardy, C. R., Simmons, M. P., y Specht, C. D. (2000). «A phylogenetic analysis of the monocotyledons based on morphological and molecular character sets, with comments on the placement of Acorus and Hydatellaceae.». En Wilson, K. L. y Morrison, D. A., ed. Monocots: Systematics and evolution. (Csiro Publ. Edición). Collingwood, Australia. PP. 17-2
- ↑ a b c Rudall, P.; Stobart, K. L., Hong, -P., Conran, J. G., Furness, C. A., Kite, G. C., y Chase, M. W. (2000). «Conside the lilies: Systatics of Liliales.». En Wilson, K. L. y Morrison, D. A., ed. Monocots: Systematics and evolution. (Csiro Publ. Edición). Collingwood, Australia. PP. 347-3
- ↑ Soltis, D. E.; Soltis, P. S., Bennett, M. D., y Leitch, I. J. (2003b). «Evolution of genome size in the angiosperms.» . Amer. J. Bot. (90): 1596-1603. doi: 10.3732/AJB.90.11.1596 . Filed from the original May 12, 2008 . Retrieved on February 25, 2008 .
- ↑ Leitch, I. J.; Soltis, D. E., Soltis. P. S., y Bennett, M. D. (2005). «Evolution of DNA amounts across land plants (Embryophyta).». Ann. Bot. 95 : 207-217. doi: 10.1093/aob/mci014 .
- ↑ Rudall, P. J.; Eastman, A. (2002). «The questionable affinities of Course (Corsiaceae): Evidence from floral anatomy and pollen morphology.». bot. J. Linnnean soc. 138 : 315-324.
- ↑ Smith, F. A.; Smith, S. E. (1997). «Structural diversity in (vesicular)-arbuscular mycorrhizal symbiosis.» . New Phytol. 137 : 373-388 . Retrieved on July 30, 2008 .
- ↑ Kubitzki, K., ed. (1998). The families and genera of vascular plants, vol 3, Monocotyledons: Lilianae (except Orchidaceae) . Berlin: Springer-Verlag.
- ↑ Kubitzki, K., ed. (2006). The families and genera of vascular plants, vol 4, Monocotyledons: Alismatanae and Commelinanae (except Gramineae) . Berlin: Springer-Verlag.
- ↑ a b c d It is f Cronquist, A. (1981). An integrated system of classification of flowering plants. . Nueva York: Columbia University Press.
- ↑ a b c Thorne, R. F. (1992). «Classification and geography of the flowering plants.». Bot. Rev. (58): 225-348.
- ↑ Lawrence, G. H. M. (1951). Taxonomy of vascular plants. (Macmillan Edición). New York.
- ↑ Huber, H. (1969). “The amen characteristics and relationships of the liliiflores.” Messages from the Munich Botanical State Collection 8 : 219-538.
- ↑ a b Huber, H. (1977). «The treatment of monocotyledons in an evolutionary systema of classification.». Plant Systematics and Evolution, Supplement first : 285-298.
- ↑ a b c d It is f Dahlgren, R. M.; Clifford, H. T., Yeo, P. F. (1985). The families of the monocotyledons. (Springer-Verlag edition). Berlin
- ↑ Chase, M. W.; Soltis, D. E., Olmstead, R. G., Morgan, D., Les, D. H., Mishler, B. D., Duvall, M. R., Price, R. A., Hills, H. G., Qiu, Y.-L., Kron, K. A., Rettig, J. H., Conti, E., Palmer, J. D., Manhart, J. R., Sytsma, K. J., Michaels, H. J., Kress, W. J., Karol, K. G., Clark, W. D., Hedrén, M., Gaut, B. S., Jansen, R. K., Kim, K.-J., Wimpee, C. F., Smith, J. F., Furnier, G. R., Strauss, S. H., Xiang, Q.-Y., Plunkett, G. M., Soltis, P. S., Swensen, S. M., Williams, S. E., Gadek, P. A., Quinn, C. J., Eguiarte, L. E., Golenberg, E., Learn, G. H., Jr., Graham, S. W., Barrett, S. C. H., Dayanandan, S., y Albert, V. A. (1993). «Phylogenetics of seed plants: An analysis of nucleotide sequences from the plastid gene rbcL . ». Ann. Missouri Bot. Gard. (80): 528-580.
- ↑ Duvall, M. R.; M. T. Clegg, M. W. Chase, W. D. Lark, W. J. Kress, H G. Hills, L. E. Eguiarte, J. F. Smith, B. S. Gaut, E. A. Zimmer, y G. H. Learn, Jr. (1993a). «Phylogenetic hypoteses for the monocotyledons constructed from rbcL sequences.». Annals of the Missouri Botanical Garden 80 : 607-619.
- ↑ a b c Chase, M. W.; Duvall, M. R., Hills, H. G., Conran, J. G., Cox, A. V., Eguiarte, L. E., Hartwell, J., Fay, M. F., Caddick, L. R., Cameron, K. M., y Hoot, S. (1995). «Molecular systematics of Lilianae.». En Rudall, P. J., Cribb, P. J., Cutler, D. F., ed. Monocotyledons: Systematics and evolution. (Royal Botanic Gardens Edición). kew. PP. 109-1
- ↑ a b c Chase, M. W.; Stevenson, D. W., Wilkin, P., y Rudall, P. J. (1995b). «Monocot systematics: A combined analysis.». En Rudall, P. J., Cribb, P. J., Cutler, D. F., ed. Monocotyledons: Systematics and evolution. (Royal Botanic Gardens Edición). kew. PP. 685-7
- ↑ a b Stevenson, D. W.; Loconte, H. (1995). «Cladistic analysis of monocot families.». En Rudall, P. J., Cribb, P. J., Cutler, D. F., ed. Monocotyledons: Systematics and evolution. (Royal Botanic Gardens Edición). kew. PP. 543-5
- ↑ Chase, M. W.; health, M. F.; Devey, D. S.; Maurin, o; rønsted, n; Davies, T. J; Pilon, y; Petersen, g; Seberg, O; Tamura, M. N.; Lange, Conny Bruru Asmussen (Faggruppe Botak); Hilu, K; Borsch, t; Davis, J. i; Stevenson, D. W.; Press, J. C.; Givnish, T. J.; Sytsma, K. J.; McPHPHERSON, M. A.; Graham, S. W.; Rai, H. S. (2006). Higher-level systematics of the monocotyledons: An assessment of current knowledge and a new classification. (pdf) . En Wilson, K. L. y Morrison, D. A., ed. «Multigene analyses of monocot relationships : a summary». Aliso (22) (Csiro Publ. Edición) (Collingwood, Australia). PP. 63-7 ISSN 0065-6275 . Magazine quotation . Retrieved on February 25, 2008 . (broken link available on the Internet Archive; see the record , the first version and the last ).
- ↑ a b Davis, J. I.; Stevenson, D. W.; petern, G.; seberg, O.; Campbell, L. M.; Freudenstein, J. V.; Goldman, D. H.; Hardy, C. R.; Michelangeli, F. A.; Simmons, M. P.; specht, C. D.; Vergara-Silva, F.; Gandolfo, M. (2004). «A phylogeny of the monocots, as inferred from rbcL and atpA sequence variation, and a comparison of methods for calculating jacknife and bootstrap values.» . Syst. Bot. (29): 467-510 . Retrieved on February 25, 2008 .
- ↑ a b c Fay, M. F.; Chase, M. W., Ronsted, N., Devey, D. S., Pillon, Y., Pires, J. C., Petersen, G., Seberg, O., y Davis, J. I. (2006). «Phylogenetics of Liliales: summarized evidence from combined analyses of five plstid and one mitochondrial loci.». Aliso (22): 559-565.
- ↑ Goldblatt, p. (1995). «the status of R. Dahlgren’s Orders lliliales and Melanthiales.». en Rudall, P. J., Crib, P. J., Cutler, D. F., y Humphries, C. J., ED. Monocotyledons: Systematics and evolution. (Royal Botanic Gardens Edición). kew. PP. 181-2
- ↑ Graham, S. W.; Zgurski, J. M., McPherson, M. A., Cherniawsky, D. M., Saarela, J. M., Horne, E. S. C., Smith, S. Y., Wong, W. A., O’Brien, H. E., Biron, V. L., Pires, J. C., Olmstead, R. G., Chase, M. W., y Rai, H. S. (2006). «Robust inference of monocot deep phylogeny using an expanded multigene plastid data set.» (pdf) . Aliso (22): 3-21 . Retrieved on February 25, 2008 .
- ↑ Hilu, K.; Borsch, T., muller, k., soltis, D. E., soltis, P. S., Savolainen, V., Chase, M. W., Powell, M. P., Alice, L. A., Evans, R., neinhuis, C., slotta, T. A. B., Rohwer, J. G., Campbell, C. S., y Chatrou, L. W. (2003). «angosperm phylogeny based on on hike sequence information.». American J. Bot. (90): 1758-1766.
- ↑ Källersjö M; JS Farris, MW Chase, B Bremer, MF Fay, ,CJ Humphries, G Petersen, O Seberg, y K Bremer (1998). «Simultaneous parsimony jacknife analysis of 2538 rbcL DNA sequences reveals support for major clades of green plants, land plants, and flowering plants.». Pl. Syst. Evol. (213): 259-287.
- ↑ Solttis de; PS SOLIS, MW Chase, Me Mort, DC Albach, M Zanis, V Savolaineen, WH Hahn, Sb Hot, MF, M AXTEL, SM SWENSEN, LM Prince, WJ Kress, KC nixon, y js frris. (2000). «Angiosperm phylogeny inferred from 18S rDNA, rbcL , and etc. sequences.» . Bot. J. Linn. Soc. (133): 381-461. Filed from the original October 2, 2007 . Retrieved on February 25, 2008 .
- ↑ Winnersen, A .; Bremer, K. (2001). «Age and biogeography of major clades in Liliales» . Amer. J. Bot. (88): 1695-1703 . Retrieved on February 25, 2008 .
- ↑ Chen, S.-C.; Yang, H., Li, S., Zhu, J., y Fu, C.-X. (2007). «Bayesian inference and its application in the molecular phylogeny of Liliales.». Acta bot. yunanana (in Chinese) 29 : 161-166.
- ↑ a b Janssen, T.; Bremer, K. (2004). «The age of major monocot groups inferred from 800+ rbcL sequences.». bot. J. Linnnean soc. 146 : 385-398.
- ↑ a b Angiosperm Phylogeny Group. (1998). «An ordinal classification for the families of flowering plants.». Ann. Fiouri bot. Gennana. 85 : 531-553.
- ↑ Zomlefer, W. B.; Williams, N. H., Whitten, W. M., y Judd, W. S. (2001). «Generic circumscription and relationships in the tribe Melanthieae (Liliales, Melanthiaceae), with emphasis on Zigadenus : evidence from ITS and trnL-F sequence data.» . Amer. J. Bot. (88): 1657-1669 . Retrieved on June 6, 2008 .
- ↑ Tamura, M. N. (1998). «liaceae». en K. Kubitzki, ed. The families and genera of vascular plants, vol. 3, Monocotyledons: Lilianae (except Orchidaceae) . Berlin: Springer-Verlag. Pp. 343-353.
- ↑ Givnish, T. J.; Pires, J. C., Graham, S. W., McPherson, M. A., Prince, L. M., Patterson, T. B., Rai, H. S., Roalson, E. H., Evans, T. M., Hahn, W. J., Millam, K. C., Meerow, A. W., Molvray, M., Kores, P. J., O’Brien, H. E., Hall, J. C., Kress, W. J., y Sytsma, K. J. (2006). «Phylogeny of the monocots based on the highly informative plastid gene NDH F : Evidence for widespread concerted convergence». Aliso 22 : 28-51.
- ↑ Chase, M. W.; Fay, M. F.; Devey, D. S.; Maurin, O; Røsted, n; Davies, T. J; Pillon, y; petern, g; seberg, O; Tamura, M. N.; Lange, Conny Bruun Asmussen (Fagggruppepe Botanic); Hilu, k; Borsch, t; Davis, J. I; Stevenson, D. W.; Pires, J. C.; Givnish, T. J.; Sytma, K. J.; McPHERSON, M. A.; Graham, S. W.; Rai, H. S. (2006). «Multigenge Analyses of Monocot Relationships : A summary.». Aliso (22): 63-75. ISSN 0065-6275 .
- ↑ Neyland, R. (2002). «A phylogeny inferred from large-subunit (26S) ribosomal DNA sequences suggests that Burmanniales are polyphyletic.». Australian Syst. Bot. 15 : 19-28.
- ↑ Bremer, K. (2000). «Early Cretaceous lineages of monocot flowering plants.». Proc. National Acad. Sci. U.S.A. 97 : 4707-4711.
Bibliography [ To edit ]
- Judd, W. S.; C. S. Campbell, E. A. Kellogg, P. F. Stevens, M. J. Donoghue (2007). «Liliales». Plant Systematics: A Phylogenetic Approach, Third edition . Sunderland, Massachusetts: Sinauer Associates. pp. 256-257. ISBN 978-0-87893-407-2 .
- Solts, D. E.; Solts, P. F., Endress, P. K., Y Chase, M. W. (2005). «Liliales» . Phylogeny and evolution of angiosperms . Sunderland, MA: Sinauer Associates. pp. 103 -104.
- Simpson, Michael G. (2005). «Liliales». Plant Systematics . Elsevier Inc. p. 180. ISBN 0-12-644460-9 ISBN 978-0-12-644460-5 .
- Stevens, P. F. (2001 en adelante). «Liliales» . Angiosperm Phylogeny Website (version 9, June 2008, and updated since then) (in English) . Retrieved on August 2, 2008 .
Suggested readings [ To edit ]
- Rudall, P.; Stobart, K. L., Hong, -P., Conran, J. G., Furness, C. A., Kite, G. C., y Chase, M. W. (2000). «Conside the lilies: Systatics of Liliales.». En Wilson, K. L. y Morrison, D. A., ed. Monocots: Systematics and evolution. (Csiro Publ. Edición). Collingwood, Australia. PP. 347-3 a lot of information including a summary of pollen variation.
- Handa, K.; Tsuji, S., y Tamura, M. N. (2001). «Pollen morphology of Japanese Asparagales and Liliales (Lilianae).». Japanese J. Hist. Bot. 9 : 85-125. for the pollen of the Japanese representatives.
- Vijayavalli, B.; P. M. Mathew (1990). Cytotaxonomy of the Liliaceae and allied families . Kerala, India: Continental Publishers. For some cytology details.
- Tamura, M. (1995). P. Hiepko, ed. The natural plant families … Volume 17 a IV. Angiospermae: Order Ranunculales. Fam. Ranunculaceae . Berlin: Duncker & Humblot. For some cytology details.
- Oganezova, G. H.; Koizumi, Y., Yokota, M., y Tsukaya, H. (2000). «Systematic position of the Trilliaceae, Smilacaceae, Herreriaceae, Tecophilaeaceae, Dioscoreaceae families and the volume and phylogeny of the Asparagales (based on seed structure).». Bot. Zhurn. 85 (9): 9-25. For ovules.
external links [ To edit ]
Recent Comments