Polymer in solution – Wikipedia
Understand the behavior of polymers in solution is important for certain applications such as cosmetic gels and creams. There is the possibility of controlling the viscosity of the product obtained and avoiding possible demixtion (phase separation). We note for example that the styrene is miscible in all proportion in the cyclohexane at 20 °C , but that when the styrene is polymized, there is no more miscibility. Different theories can explain these phenomena.
We model a polymer chain by a set of n links (monomer units) of volume a. The average number of links is linked to the degree of polymerization and the molar mass of the polymer. It is in solution in a solvent with a volume fraction or a known concentration.
Solvant polymer mixture [ modifier | Modifier and code ]
It is hypothesized that the molar volume of monomers units is equal to that of solvent molecules.
The mixture can be represented as this:

At fixed temperature and pressure, the free mixture of mixture must be negative for the mixture to take place.
In the case of a regular solution, statistical thermodynamic calculations make it possible to estimate this free enthalpy:
with
The Boltzmann constant, t la targeure in Kelvins,
the volume fraction and
A dimensionless parameter.
For a polymer in a solvent, Flory posed [ first ] :
With n the average number of monomers per chain units.
This free enthalpy is actually the sum of two terms [ 2 ] :
Or :
- is the mixture of mixture;
- is the mixture of mixture, it is the latter which is decreased by factor N.
When n or the molar mass (it is equivalent) increases, entropy decreases, so disorder as well. There are indeed fewer possibilities to distribute the monomers units and the solvent molecules on the above grid when the chains are longer. The result is a free enthalpy of mixing more difficultly negative when the polymer has a stronger molar mass. This is in accordance with the precipitation that can be observed when you synthesize a polymer in solution, therefore in a solvent.
FLORY parameter [ modifier | Modifier and code ]
The parameter
which intervenes in the expression of the enthalpy and therefore in that of the free enthalpy, makes it possible to take into account the interactions between the polymer and the solvent. In this model [ 3 ] , [ 4 ] ,
Or :
- Z is the number of first neighbors of monomeric units or solvent molecules;
- is the interaction energy between monomer and solvent unit;
- is the energy of interaction between monomers units;
- is the interaction energy between solvent molecules.
This is Flory’s parameter, which plays an important role in the evolution of the free enthalpy:

From a certain value of
, the curve has two inflection points and it is possible to draw a double tangent [ 5 ] . It is a sign of a separation in two phases like this [ first ] :

We can calculate this critical value of Flory parameter [ first ] , noted
and obtained for
.
To summarize, the demixion appears when the Flory parameter exceeds a critical value which depends on the molar mass. This can also be understood by comparing the interactions that appear in the expression of this parameter. When
is tall,
dominated. This attractive energy is of course negative and when it is large, it decreases in absolute value: the attraction between polymer and solvent weakens. The links in polymer chains are attracted to each other and the polymer will be less and less solubilized.
We can access this flory parameter experimentally by osmotic pressure measurements for example.
State diagram -T [ -T”>modifier | -T”>Modifier and code ]
For a polymer/solvent system, if the Van der Waals of London type interactions dominate (typically in the apolar environment), we can show that
varies as the reverse of the temperature. At low temperature, the Flory parameter will be large and we will have a demixion, and vice versa.
On the other hand when the hydrogen bonds dominate (polar environment),
Increases with temperature and we therefore have the opposite situation. At high temperature the thermal agitation breaks the hydrogen bonds which linked polymer to the solvent and the phase separation takes place.
To obtain these phase diagrams, we can start from tubes containing different molary fractions in polymer and observe when each tube becomes troubled when cooled from a temperature for which we mix [ 6 ] . The disorder is due to the diffusion of light in all directions by the particles which appear when the phase separation occurs:

Hildebrand approach, solubility parameter [ modifier | Modifier and code ]
We define the cohesion energy of the system and the cohesive energy density which is obtained by dividing the first by the molar volume [ 6 ] :
- .
The solubility parameter is then equal to the square root of this C density [ 6 ] :
- .
The solubility parameter values are tabulated for many solvents and many polymers [ 7 ] . We can show that the Flory parameter seen previously can be written [ 8 ] :
With R the perfect gas constant.
If we are looking for a good solvent for a given polymer, we must therefore seek a solubility parameter that is close to that of the polymer, to minimize the Flory parameter. We can thus eliminate many solvents for a given polymer, but even if Hildebrand’s parameters are close, the solubility is not ensured [ 9 ] .
We can also calculate the solubility parameter as this [ 5 ] :
where G (i) is the molar attraction constant of group I [ 7 ] .
Let two compartments are separated by a semi permeable membrane:

The right compartment contains the polymer in solution in a solvent, while the left compartment contains only the solvent. We observe a variation in the pressure between the 2 compartments. More specifically, polymer chains exercise a force on the wall. This strength per unit of surface is called osmotic pressure . It can be calculated for a few hypotheses from the mixture of mixture seen previously.
If the volume fraction in polymer is not too large, we get:
- .
We realize that the value of the Flory parameter here still plays a role:
The Flory parameter
varies with temperature. When it takes the limit value 1/2, the associated temperature bears the name of “Théta temperature”. This particular temperature can often be in tables for a polymer/solvent torque. For example, cyclohexane is a polystyrene tea solvent [ 5 ] to 307.2 K.
This theory introduces the concept of excluded volume: there is a repulsion between chains, of a geometric nature, which prohibits the presence of the center of mass of another polymer chain around a given chain.

The previously calculated florry parameter is changed [ 11 ] :
- .
With a polymer – solvent torque, we can associate a temperature
and a parameter
it Entropic parameter , both independent of the temperature [ 11 ] :
- .
Knowing the critical temperature
(of the appearance of the disorder) for samples of a polymer of different molar masses, we can graphically determine the temperature
and the entropic parameter.
The excluded volume (U), the Flory parameter and the second viriel coefficient of the Polymer/Solvant A2 couple are linked by the following relations:
- .
Na is the number of avogadro, n the number of segments, m the molar mass and has the volume of a monomer unit.
When the flory parameter is less than 0.5, the excluded volume increases: there is extension of the chains and we are in a good solvent diet. In bad solvent it is the opposite, there is a contraction of the channels. We can experimentally obtain the volume excluded by osmometry [ twelfth ] .
There are different regimes, depending on the concentration and mass of the polymer in solution. For entropic reasons, polymer channels are not unfolded but they contract on themselves to form balls.
Of the diluted diet with a semi -diluted diet [ modifier | Modifier and code ]
Be polymer balls in solution. When the concentration (or the volume fraction is increased which amounts to the same), the distance between balls decreases. From a moment, there is contact between the latter and we call Critical recovery concentration the associated concentration. It marks the transition between the diluted regime and the Semi diluted diet . It is still possible to increase the concentration in the semi -diluted diet, because the fraction of polymer contained in a ball is really low.

We can calculate the critical recovery concentration using the following relation [ 3 ] , [ 13 ] :
- .
The volume fraction depends on the number of segments of the channels to power -4/5.
Giration radius in diluted regime [ modifier | Modifier and code ]
This distance has an influence on the physicochemical properties of the system.
In good solvent [ 6 ] :
- ,
With RG the gimal radius of the balls and n the number of segments of the chain which constitutes the ball and is the size of these segments.
Solvent
:
- .
We can understand this difference of exponent by taking into account the fact that by good solvent
, the repulsive interactions between segments of balls dominate. The balls swell and their radius of giration increases.
Solvent
, there are different models to calculate the giration radius:
- Random walking model [ 2 ] : [ 6 ] , [ 14 ] :
- is the angle of Valence, taken according to this convention:

- Embarrassed rotation: we take into account the interactions between molecular groups.
- is the internal rotation angle or dihedral angle. You can see like a rigidity coefficient.
If we are no longer in conditions
, we can introduce a coefficient of volume expansion like this [ 15 ] :
- Characteristic size in semi -diluted regime [ modifier | Modifier and code ]
When the balls interpenetrate, we can no longer really speak of a radius of gimal, since we cannot distinguish the balls. We define the average distance between tangle points, noted
. This quantity is equal to
When we are at the critical concentration of recovery. It then decreases when the concentration increases. The properties of the system depend on this quantity in the semi -diluted diet. We can show that [ 2 ] :
- As a good solvent.
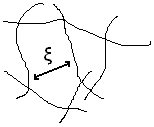 is a correlation length, or network mesh.
is a correlation length, or network mesh.The tangled diet [ modifier | Modifier and code ]
It is possible when the mass exceeds a critical mass of tangle MC. It can appear in the semi -diluted diet, even in the concentrated diet (when the concentration is even increased).
In the tangled diet, the movement of the chains is very embarrassed by that of the other chains. Its movement is described by the reputation model. The viscosity then depends on the concentration of 3.75 power and the mass in the cube. In a non -tangled diet, the viscosity depends on the concentration of only 2.5 power and the chains have a so -called “rouse” dynamic. This more or less large effect of concentration makes it possible to play on the viscosity of the system in a significant way and can be used when making polymer gels.There are different techniques to study polymers in solution:
- Colligative thermodynamic methods:
- Viscosimetric methods:
- Access to viscosity, the average viscosimetric molar mass [ 6 ] (Mn
- Access to viscosity, the average viscosimetric molar mass [ 6 ] (Mn
- hydrodynamic techniques:
- These, access to mass molar masses in mass and moles;
- light diffusion techniques:
- Menno A. van Dijk and André Awake , Concepts of polymer thermodynamics , CRC Press, , 209 p. (ISBN 1-56676-623-0 And 9781566766234 , read online )
- D ESPINAT , Application of light diffusion techniques, X -rays and neutrons to study colloidal systems , Paris, Technip editions, , 131 p. (ISBN 2-7108-0617-7-7 And 9782710806172 , read online )
- Hans-Georg Elias , Macromolecules : Volume 3 : Physical Structures and Properties , Wiley-VCH, , 699 p. (ISBN 978-3-527-31174-3 And 3-527-31174-2 , read online )
- Some authors define it with Z-2 rather than Z.
- J. M. G. Cowie , Polymers : chemistry and physics of modern materials , CRC Press, , 436 p. (ISBN 0-7487-4073-2 And 9780748740734 , read online )
- Hans-Henning Cock , Nicole Heymans , Christopher-John Plummer and stone Decroly , Polymers’ materials: mechanical and physical properties, [Principles of implementation] , Lausanne/Paris, ppur Polytechnic presses, , 657 p. (ISBN 2-88074-415-6 And 9782880744151 , read online )
- Hildebrand parameter values and molar attractions constants
- Jean Vidal , Thermodynamics: Application to chemical engineering and the petroleum industry , Paris, Technip editions, , 500 p. (ISBN 2-7108-0715-7-7 And 9782710807155 , read online )
- Andreĭ aleksandrovich Askadskiĭ , Physical properties of polymers : prediction and control , CRC Press, , 336 p. (ISBN 2-88449-220-8 And 9782884492201 , read online )
- (in) MICHIO Kurata ( trad. Japanese), Thermodynamics of polymer solutions , Chur/London/New York, Taylor & Francis, , 294 p. (ISBN 3-7186-0023-4 And 9783718600236 , read online )
- Yuri S. Lipatov and anatoly E. Nesterov , Thermodynamics of polymer blends , CRC Press, , 450 p. (ISBN 1-56676-624-9 And 9781566766241 , read online )
- Paul C. Hiemenz , Polymer chemistry : the basic concepts , CRC Press, , 738 p. (ISBN 0-8247-7082-X And 9780824770822 , read online )
- Some authors define it with a 0.74 multiplicative coefficient in the numerator.
- If we take another convention to define the angle, the formula is reversed.
- English lesson
- Characteristic size in semi -diluted regime [ modifier | Modifier and code ]

 The Boltzmann constant, t la targeure in Kelvins,
The Boltzmann constant, t la targeure in Kelvins,  the volume fraction and
the volume fraction and  A dimensionless parameter.
A dimensionless parameter. 










 and obtained for
and obtained for  .
. 





 and a parameter
and a parameter  it Entropic parameter , both independent of the temperature
it Entropic parameter , both independent of the temperature 
 (of the appearance of the disorder) for samples of a polymer of different molar masses, we can graphically determine the temperature
(of the appearance of the disorder) for samples of a polymer of different molar masses, we can graphically determine the temperature 



 , the repulsive interactions between segments of balls dominate. The balls swell and their radius of giration increases.
, the repulsive interactions between segments of balls dominate. The balls swell and their radius of giration increases. 




 . This quantity is equal to
. This quantity is equal to  When we are at the critical concentration of recovery. It then decreases when the concentration increases. The properties of the system depend on this quantity in the semi -diluted diet. We can show that
When we are at the critical concentration of recovery. It then decreases when the concentration increases. The properties of the system depend on this quantity in the semi -diluted diet. We can show that 
Recent Comments