Aziridine — Wikipedia
A wikipedia article, free l’encyclopéi.
| Aziridine | |
  |
|
| Identification | |
|---|---|
| UICPA name | aziridine |
| Synonyms |
azacyclopropane |
| N O CAS | |
| N O Echa | 100.005.268 |
| N O THIS | 205-793-9 |
| Pubchem | 9033 |
| Chebi | 30969 |
| SMILES | |
| Inches |
|
| Appearance | Colorful and clear oily liquid [ first ] |
| Chemicals | |
| Formula | C 2 H 5 N [Isomers] |
| Molar mass [ 3 ] | 43.067 8 ± 0.002 2 g/mol C 55,78 %, H 11,7 %, N 32,52 %, |
| Dipolar moment | 1.90 ± 0.01 D [ 2 ] |
| Physical properties | |
| T° fusion | −78 °C [ 4 ] |
| T° boiling | 56 °C [ 4 ] |
| Volumic mass | 0.832 1 g · cm -3 To 20 °C [ 5 ] |
| T° d’auto-inflammation | 320 °C [ 6 ] |
| Flash point | −13 °C [ 6 ] |
| Explosiveness limits in the air | 3.6 – forty six %vol sixty four – 820 g · cm -3 [ 6 ] |
| Saturating steam pressure | 213 mbar To 20 °C 333 mbar To 30 °C 780 mbar To 50 °C [ 6 ] |
| Thermochimie | |
| D f H 0 liquid | 91.9 kJ · mol -first [ 4 ] |
| C p |
|
| PCI | −1 591,36 kJ · mol -first [ 4 ] |
| Precautions | |
| Simdut [ 9 ] | |
   B2, D1A, D2a, AND, |
|
| NFPA 704 [ ten ] | |
|
|
|
| Directive 67/548/EEC | |
|
|
|
| Transport [ 6 ] | |
|
|
|
| Classification du CIRC | |
| Group 2B: perhaps carcinogenic for humans [ 8 ] | |
| Ecotoxicology | |
| DL 50 | 15 mg · kg -first (rat, oral) 3.5 mg · kg -first (rat, i.p.) 4 mg · kg -first (Mouse, I.P.) [ 11 ] |
| Smell | down : 2 ppm [ twelfth ] |
|
|
|
| IS units and CNTP , unless otherwise stated. | |
| modifier |
|
L’ aziridine (n.f.) or azacyclopropane is the cyclic organic compound of raw formula c 2 H 5 N. It is also the parent compound of the aziridines and the functional group which corresponds to a heterocycle on three sides composed of an amine and two methylene groups [ 13 ] , [ 14 ] .
Aziridines can be prepared in organic chemistry in many ways.
Cycle of haloamines and amino-alcohols [ modifier | Modifier and code ]
An amine group can move an adjacent (β) halogenure to an intramolecular nucleophilic substitution reaction to form an aziridine. Amino-alcohols have the same reactivity but it is first necessary to convert the hydroxide groups to better starting groups, for example in Tosylate. The cyclization of an amino-alcohol is called synthesis of wenker (in) (1935) and one of haloamine, the Gabriel ethylenimine-method (1888) [ 15 ]
Nitrene addition [ modifier | Modifier and code ]
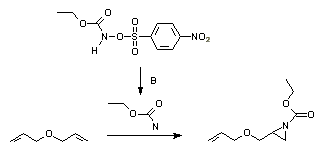
The addition of a nitrene on an alcene is a well -established method for the synthesis of the aziridines. Photolys or thermolyzes of nitrums are a good path to generate nitrenes. These can also be generated in situ of iodobenzene diacetate and sulfonamated or sulfamates, or ethoxyarbonylnitrene of an N-Sulfonyloxy precursor [ 16 ] :
Decomposition of triazolines [ modifier | Modifier and code ]
Under thermal treatment or by photolysis of a triazoline, nitrogen is expelled and remains an aziridine [ 17 ] . The required triazoline is obtained from a cycloadition reaction of an azoture on an alcene.

Via the opening of an epoxide [ modifier | Modifier and code ]
A method consists of a cycle opening reaction of epoxide with sodium azoture followed by an organic reduction in the azoture obtained with the triphenylphosphine by expulsion of gaseous nitrogen [ 18 ] :
Another method is the reaction to open an epoxide by an amine followed by the closing of the cycle by a reaction from Mitsunobu [ 19 ] .
Reaction of an oxime with a grignard reagent [ modifier | Modifier and code ]
Hoch-Campbell synthesis synthesis describes the synthesis of Aziridines by the reaction of certain oximes with Grignard reagents [ 20 ] , [ 21 ] :
Structure [ modifier | Modifier and code ]
The corners of the bonds in the aziridine are around 60 ° which considerably less than the angle of 109.5 ° Normally found in hydrocarbons or linear and non -constrained amines. These angles are however comparable to those found in cycles cycles or oxirane and correspond to the same angular constraints in the cycle: the connections in this type of cycle can be explained by invoking a banana connection model. Aziridine is less basic than an acyclic and aliphatic amine with a pka of 7.9 for conjugated acid which is due to an increase in the S character in the free pair of nitrogen. The angular constraints increased in the aziridine are also responsible for the increase in the energy barrier for the inversion of the nitrogen. This barrier is high enough in the aziridines to be able to separate from invert Like the CIS and Trans inverts of the N-CHLORO-2-METHYLAZIRIDINE.
Reactions [ modifier | Modifier and code ]
Nucleophilic cycle opening [ modifier | Modifier and code ]
Aziridines are substrates sensitive to the cycle opening reactions with many nucleophiles because of molecular constraints in the cycle. Alcoholisse and ammonialysis are typically the opposite reactions of cycling. Other effective nucleophiles are nucleophilic carbons as in organolithian reagents or organocuprates such as Gilman’s reagent.
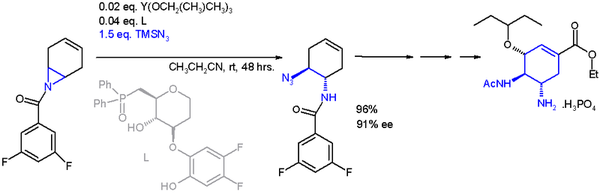
An important application of an asymmetrical synthesis cycle opening reaction is that with the TRIMETHYLSILYLAZOTURE TMSN 3 and an asymmetrical ligand [ 22 ] :
The catalyst is built on yttrium and three Isopropyloxy substituents, ligand is a phosphine oxide. With an enantiomeric excess of 91% EE, this reaction allows a total synthesis of the oseltamivir (Tamiflu).
Other reactions [ modifier | Modifier and code ]
Non-n-substitled aziridines can be opened by alkenes in the presence of a strong acid like B (C 6 F 5 ) 3 [ 23 ] Some n-substitled aziridines and with attractive electronic groups on the two carbon atoms form azomethine ylures by an electrocyclic reaction (in) cycle opening. These ylures can be trapped with a suitable dipolarophile by a 1.3-dipolary cycloadition.
The toxicology of each specific aziridine depends on its own structure and activity which participates in the general characteristics of the Aziridines group. Being electrophilic, the aziridines are likely to attack and see their cycle opened by endogenous nucleophiles such as the Nucleic DNA bases which corresponds to a mutagenic potential [ 24 ] , [ 25 ] , [ 26 ] , and can also induce in certain cases an antitumoral activity [ 27 ] .
Thus inhalation and direct contact with the aziridines are prohibited. Some reports note that even gloves used normally in chemistry do not prevent skin infiltration of the aziridines. It is therefore important that users check the gloves’ impregnation times and scrupulously pay attention to avoiding contamination when removing gloves.
The International Cancer Research Agency (IARC =International Agency for Research on Cancer ) reviewed the aziridine compounds and classified them as possible human carcinogen (Group 2B) [ 28 ] . By carrying out a global evaluation, the IARC working group has taken into account the fact that the aziridines are direct alkylating agents which are mutagenic in a wide range of test systems and form DNA adduits which are promoted.
Aziridines cause irritating effects on mucous membranes such as eyes, nose, respiratory system and even skin. Aziridines penetrate quickly through the skin in case of contact. They can cause allergic dermatoses and hives or give occupational asthma.
- Aziridine.pdf sur IARC
- (in) David R. Like, Handbook of chemistry and physics , Boca Raton, CRC, , 89 It is ed. , 2736 p. (ISBN 978-1-4200-6679-1 And 1-4200-6679-X ) , p. 9-50
- Molar mass calculated after ‘ Atomic weights of the elements 2007 » , on www.chem.qmul.ac.uk .
- (in) « Ethylenimine » , on NIST/WebBook , consulted on July 22, 2009
- CRC Handbook of Chemistry and Physics, 59th ed, (ISBN 978-0-8493-0549-8 )
- “Ethyleneimine” input in the chemical product database Achievement IFA (German organization responsible for occupational safety and health) ( German , English ), access on July 22, 2009 (Javascript required)
- (in) Carl L. Washs, Handbook of Thermodynamic Diagrams , vol. 1, Huston, Texas, Gulf Pub. Co., (ISBN 0-88415-857-8 )
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, ‘ Global carcinogenicity assessments for humans, 2b group: perhaps carcinogens for humans » , on http://monographs.iarc.fr , CIRC, (consulted the )
- “Ethylene Imine” in the chemical product database Reptox of the CSST (Quebec organization responsible for occupational safety and health), consulted on July 22, 2009
- UCB University of Colorado
- (in) ‘ Aziridine » , on Chedplus , consulted on July 22, 2009
- ‘ Ethyleneimine » , on hazmap.nlm.nih.gov (consulted the )
- Heterocyclic chemistry , T.L. Gilchrist. (ISBN 0-582-01421-2 )
- Epoxides and aziridines – A mini review , Albert Padwaa and S. Shaun Murphreeb; Arkivoc (JC-1522r), p. 6-33 . online article
- Gabriel Ethylenimine Method
- Addition reactions of ethoxycarbonylnitrene and ethoxycarbonylnitrenium ion to allylic ethers M. Antoniotta lored et al .; TetraheDron litters, 1984, Vol. 25 (38), p. 4271-4274 . Résumé
- Triazoline photodecomposition : The preparation of aziridines , P. HIRD; Tetrahedron, 1968, flight. 24 (6), p. 2757-2765 . DOI 10.1016/S0040-4020(01)82547-3 .
- Readily Available Unprotected Amino Aldehydes , Ryan Hili and Andrei K. Yudin; J. AM. Chem. Soc., 2006, Vol. 128 (46), p. 14772-14773 . DOI 10.1021/ja065898s .
- Aravinda B. Pulipaka, Stephen C. Bergmeier.; Synthesis, 2008, vol. 9, p. 1420-1430
- Hoch, comppt. REND., 196, 1865 (1934); (a), Ibid., Aos, 799 (1936); (e), Ibid., 204, 354, 353 1937).
- The action of Grignard reagents on oximes. I. The action of phenylmagnesium bromide on mixed ketoximes , Kenneth N. Campbell, James F. Mckenna; J. Org. Chem., 1939, vol. 4(2), p. 198-205 . DOI 10.1021/JO01214A012 .
The reaction of Grignard reagents with oximes. II. The action of aryl grignard reagents with mixed ketoximes , Khanth N. Campbell, BarbaBa Snapping Campbell, Elamer Paul Chap; J. Org. CHEM., 1943, flight. 8 (1), p. 99-102 . DOI 10.1021/jo01189a015 .
The action of Grignard reagents on oximes. III. The mechanism of the action of arylmagnesium halides on mixed ketoximes. A new synthesis of ethyleneimines , Kenneth N. Campbell, Barbara K. Campbell, James F. Mckenna, and Elmer Paul Chaput; J. Org. Chem., 1943, p. 103-109 . DOI 10.1021 / JO01189A016 - De Novo Synthesis of Tamiflu via a Catalytic Asymmetric Ring-Opening of meso-Aziridines with TMSN3 , Yuhei Fay et al .; J. AM. Chem. SOC., 2006, Vol. (19), p. 6312-6313 . DOI 10.1021 / ja061696k .
- Aravinda B. Pulipaka and Stephen C. Bergmeier.; J. ORG. Chem., 2008, vol. 73. p. 1462-1467
- Occupational respiratory and skin sensitization caused by polyfunctional aziridine hardener , L. Kanerva et al.; Clinical & Experimental Allergy, 1995, vol. 25 (5), p. 432–439 .
- Skin and respiratory allergic disease caused by polyfunctional aziridine , Sartorelli P et al.; With low., 2003, vol.94 (3), p. 285-95 .
- Agents, old and new, causing occupational asthma , MAPP CE; Occupate. Approximately. Med., 2001, vol. 58, p. 354-60
- Giorgi-Renault, S., Renault J., Baron M., Gebel-Servolles, P., Delic, J., Cros S., Paoletti C., Heterocyclic quinones XIII. Dimerization in the series of 5,8-quinazolinediones: Synthesis and anti tumor effects of bis(4-amino-5,8-quinazolinediones), Chem. Pharm. Bull., 36 (10), 3933-3947 (1988).
- Volume 9.pdf, Some Aziridines,…








Recent Comments