Benzoate of Denatonium — Wikipedia
| Benzoate de dénatonium | |
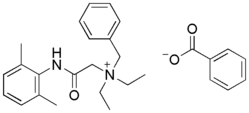 |
|
| Identification | |
|---|---|
| UICPA name | phenylmethyl-[2- [(2,6-dimethylphenyl)amino]- 2-oxoethyl]-diethylammonium benzoate |
| Synonyms |
Lidocaine benzyl benzoate |
| N O CAS | |
| N O Echa | 100.020.996 |
| N O THIS | 223-095-2 |
| Pubchem | |
| SMILES |
|
| Inches |
|
| Appearance | Solid white odorless [ first ] |
| Chemicals | |
| Formula | C 28 H 34 N 2 O 3 [Isomers] |
| Molar mass [ 2 ] | 446.581 2 ± 0.026 1 g/mol C 75,31 %, H 7,67 %, N 6,27 %, O 10,75 %, |
| Physical properties | |
| T° fusion | 168 °C [ 3 ] |
| Solubility | 45 g · l -first in water ( 20 °C ) [ first ] Soluble in ethanol, methanol, butanol, isopropanol and chloroform [ 4 ] . |
| Precautions | |
| Directive 67/548/EEC | |
 Xn |
|
| Ecotoxicology | |
| DL 50 | 584 mg · kg -first (Rats, oral) [ 3 ] 508 mg · kg -first (Rabbits, oral) [ 3 ] |
| Related compounds | |
| Other anions | Saccharinate of Denatonium, Denatonium chloride |
| Other compounds |
Lidocaine |
|
|
|
| IS units and CNTP , unless otherwise stated. | |
| modifier |
|
The benzoate de dénatonium is a benzoic salt of denatonium with a very bitter taste. It was discovered in 1958 during research on local anesthetics. It is used as repellants to prevent the ingestion of household products and antigels, in nail polish in the fight against onychophagy and is used to distort ethanol. This artificial compound is as bitter as the Quassine, the most bitter known natural compound.
Discovery [ modifier | Modifier and code ]
Denatonium benzoate was discovered in 1958 by an Scottish pharmaceutical laboratory named MacFarlan Smith in Edinburgh [ 5 ] . This discovery occurred during research to improve the anesthetic properties of Lidocaine.
The laboratory already extracted brucine (bitter compound) in order to distort the alcohols, but the benzoate of denatonium being more bitter and devoid of toxicity, the latter quickly became the ideal denaturing. It is also the name of its use of denaturing that this compound takes its name, the trade name being linked to the English word bitter signifier amer [ 5 ] . Another of the first Bitrex applications as an aversion agent was a cream designed to prevent pigs from eating their neighbor’s tail [ 5 ] .
Legislation [ modifier | Modifier and code ]
As early as 1960, denatonium benzoate, under the brand Bitrex , is authorized in the United States and the United Kingdom in perfumes, perfumery, cosmetics and other industrial uses. Since then, he has been recognized as a distorting agent and aversion agent in more than 40 countries [ 5 ] .
In 1993, he was authorized in the European Union as a denaturing of alcohol for the exemption from the right of excise [ 6 ] .
In 1995, French law, by decree, made it compulsory the addition by the industrialists of Benzoate de Denatonium or another repellent agent in the antigels and heat transfer fluids containing monoethyleneglycol monoethylene [ 7 ] .
Since 1999, it is compulsory to use it in certain pesticides [ 5 ] .
Structure [ modifier | Modifier and code ]
Denatonium benzoate is a summary salt comprising an anion (benzoate) associated with a cation (a quaternary ammonium). The cation has a structure similar to local anesthetic, lidocaine, of which it differs only by the addition of a benzyl group on the tertiary amine.
Denatonium can be associated with other anions in the form of salt such as the saccharinate ion to form the saccharinate of Dénatonium [ 8 ] or the chloride ion to form distant chloride [ 9 ] .
Physico-chemical properties [ modifier | Modifier and code ]
Denatonium benzoate is soluble in water [ first ] , ethanol, methanol, butanol, isopropanol and chloroform [ 4 ] ..
The melting temperature of denatonium benzoate is 168 °C .
Sensory property [ modifier | Modifier and code ]
Denatonium benzoate has a detection threshold in ten ppb and a threshold for recognizing the bitterness of 0.05 ppm , which makes it as bitter as the Quassine, the most bitter known natural compound with a threshold for recognition of the bitterness of 0.06 ppm [ ten ] .
The nature of the anion with which denatonium salt is associated modifies the concentration of the threshold for recognition of bitterness. Thus, for the saccharinate of denatonium and the chloride of denatonium, it is of 0.01 ppm And 0.1 ppm respectively [ 9 ] .
Discalnut benzoate is really very bitter to ten ppm [ 11 ] .
Dermatology [ modifier | Modifier and code ]
Destrique benzoate is considered harmless to skin in cosmetic products at the doses used ( 0.000 6 % ) [ 11 ] .
Discalitating benzoate is mainly used for its bitter taste as denaturing, repellant agent, aversion agent or fitting.
In the European Union, denatonium benzoate is used as denaturing in alcohols, mainly for the exemption from the right to accuse [ 6 ] .
It is also used to discourage the consumption of toxic alcohols such as methanol and glycol ethylene. In France industrialists add a minimum of 20 ppm Denatonium benzoate in products containing glycol ethylene, this concerns antigels, heat transfer fluids and laundry laundry products [ 7 ] .
Destrust benzoate is recommended in the fight for the protection of children and the decrease in household accidents due to the absorption of household products (detergents, detergent, softening products), cosmetics, perfumes and bath products (shampoo). The intense bitterness of denatonium benzoate forces the child to spit out the product immediately after putting it in the mouth [ twelfth ] .
Discalite benzoate is used as an additive in products to combat rodents (Campagnol, Ragondin, Rat, Mouse) and Cervivés and deer [ 13 ] .
Discalnut benzoate is also used in a bitter nail polish to combat the mania to bite your nails (onychophagy).
The cartridges of the Nintendo Switch console are coated, so that children do not swallow them [ 14 ] ; They are indeed very small.
- (in) “Denatonium benzoate” input in the chemical product database Achievement IFA (German organization responsible for occupational safety and health) ( German , English ) (Javascript required)
- Molar mass calculated after ‘ Atomic weights of the elements 2007 » , on www.chem.qmul.ac.uk .
- (in) Chedplus, ‘ Denatonium benzoate – RN: 3734-33-6 » , on chem.sis.nlm.nih.gov , U.S. National Library of Medicine (consulted the )
- Specifications of denatonium benzoate on aisle
- Anonymous, ‘ The origins of Bitrex® » , on http://www.bitrex.com (consulted the )
- European Commission « Regulation (EC) No 3199/93 of the Commission, of November 22, 1993, relating to the mutual recognition of processes for the complete denaturation of alcohol for the exemption from the right of excise », Official newspaper , n O L 288, , p. 12-15 ( read online ) [PDF]
- AND Balladur « Decree No. 95-326 of March 20, 1995 relating to security obligations concerning the distribution of certain liquids based on monoethyleneglycol », Jorf , n O 72, ( read online )
- (in) Chedplus, ‘ Denatonium saccharide – RN: 90823-38-4 » , on chem.sis.nlm.nih.gov , U.S. National Library of Medicine (consulted the )
- (in) Consumer Product Safety Commission – Study on Aversive Agents , 11/92 Final Report. [PDF]
- (in) YPS Bajaj , Is Scragg a not Allan , ‘ Medicinal and Aromatic Plants : XXI Quassia amara (Surinam Quassia): In vitro culture and the production of quassin » , Biotechnology in agriculture and forestry , Springer, vol. 26, , p. 316-326 (ISBN 9783540563914 )
- (in) Cosmetic Ingredient Review Expert Panel, ‘ Final report of the safety assessment of Alcohol Denat., including SD Alcohol 3-A, SD Alcohol 30, SD Alcohol 39, SD Alcohol 39-B, SD Alcohol 39-C, SD Alcohol 40, SD Alcohol 40-B, and SD Alcohol 40-C, and the denaturants, Quassin, Brucine Sulfate/Brucine, and Denatonium Benzoate » , International Journal of Toxicology , vol. 27, n O S1, , p. 1-43 (DOI 10.1080/10915810802032388 , résumé )
- Domestic accident prevention institute, ‘ A prevention product, very effective and little known: the “bitrex”. » , Ipad sheet partner , on http://www.ipad.asso.fr , IPAD (consulted the )
- Ministry of Agriculture and Fisheries, ‘ Substance active: Benzoate de denatonium » , on http://e -phy.agriculture.gouv.fr (consulted the )
- ‘ Do not try, do the switch cartridges really taste? », iGeneration , ( read online , consulted the )
Related articles [ modifier | Modifier and code ]
external links [ modifier | Modifier and code ]
Recent Comments