Imiquimod – Wikipedia
Imiquimod (in the experimental phase known with the abbreviation R-837 ) is a drug that acts as an immunomodator, that is, as a modifier of the immune response. In Italy it is sold by the pharmaceutical company Meda AB with the commercial name of Aldara in the pharmaceutical form of cream containing 5% of the active ingredient, and also with the commercial name of Immunocare .
IMIQUIMOD can be synthesized starting from the 4-cloro-3-nitrochinoline compound: 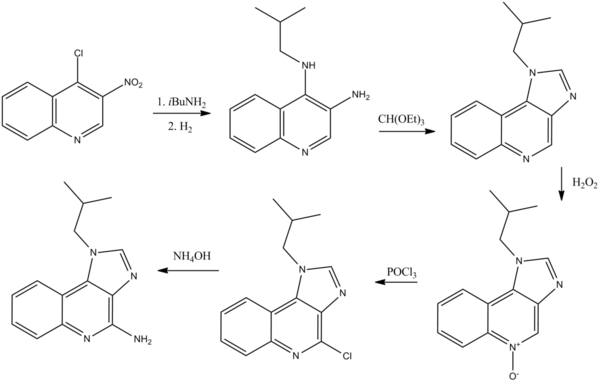
The mechanism of action of the drug is not yet completely clarified. It is known that on immune cells there is a specific membrane receptor for IMIQUIMOD, probably the Toll-like Receptor 7 (TLR7), whose interaction with the molecule determines a complex response from the immune system, [first] which probably determines the activation of Type 1 T -Helper lymphocytes (TH1). [2] The cells activated by IMIQUIMOD via TLR-7 begin to secrete numerous types of cytokines, mainly interferon-α (Ifn-α), interleuchin-6 (il-6) and the factor of tumor-α necrosis (TNF-α). [3]
There is also an evidence that IMIQUIMOD, when applied to the skin, can lead to the activation of the Langerhans cells, which later migrate to local lymph nodes to activate the adaptive immune system. [4]
Other types of cells activated by IMIQUIMOD include Natural Killer cells, macrophages and B lymphocytes. [4]
Further studies suggest that IMIQUIMOD can exercise its effects by determining an increased expression (Opeguulation) of the receptor (Ogfr) of the growth factor of opioids (OGF; [MET (5)]-Encephalin). A block of the function of this OGFR receptor using a short interfering RNA (Sirna or Rna interferent patents ) has led to the loss of any antiproliferative effect by IMIQUIMOD. [5] [6]
In adult subjects it is used in the treatment of some diseases of the skin such as external acuminated condition of the genital and perianal region and in Bowen’s disease. [7] It is also used in the treatment of surface basallular carcinomas and limited dimensions.
It also finds an indication in the therapy of areas of attinical keratosis present on the face and skull when it is not possible or appropriate to resort to other therapeutic options, for example cryotherapy, because the number or size of the lesions can limit its effectiveness or acceptability by the patient.
Often the treatment with Imequimod Crema follows surgery, as surgical therapy seems to offer a better possibility of effectively treating some forms of skin cancer.
IMIQUIMOD has also been tested for the treatment of contagious mollusc. Two large randomized and controlled studies were performed, which, however, have found no evidence of the effectiveness of the molecule in the treatment of children with contagious mollusk- also during the study, different adverse effects were also noted.
IMIQUIMOD has also been tested for the treatment of intraepithelial neoplasia vulvar, [8] of common warts scarcely responding to other therapies and vaginal intraepithelial neoplasm. [9]
Relevant aesthetic results have been obtained with the treatment of basocellular carcinoma and squamous cell carcinoma in situ, especially if extended to large skin aerial, but the morbidity and discomfort related to the treatment can be serious.
The treatment rarely can lead to a certain degree of permanent scars.
Focal tumor relapse have been observed after treatment with imiquimod, but seem to be susceptible to surgical excision.
IMIQUIMOD also has the characteristic of highlighting injuries that at first were subclinical and facilitating their elimination by the immune system: [ten] These areas of new emerging injuries following the therapy can be highlighted with the simple execution of photographs of the attinical keratosis and the patient’s basic basic basic carcinomas, first, during and after treatment. [11]
In the clinical studies carried out the adverse reactions reported more frequently, and in a likely relationship to treatment with IMIQUIMOD Crema, they occur against the application area. The frequency of these reactions is variable depending on the basic disease (external genital effects, basalcellular carcinoma, attinical keratosis) for which the treatment begins.
- External genitalia effects
Patients commonly report fatigue, nausea, headache, muscle pain.
In addition to these events, local skin reactions have also been recorded such as the appearance of erythema (61%of patients), skin erosion (30%), bruising or desquamation (23%) and localized edema (14%).
- Basocellulare superficiale carcinoma
The disorders referring more frequently consist of dorsal pain, lymphadenopathy, and sometimes headache and flu disorders.
The most disturbing side effects are the local ones and among these serious erythema (31%of the subjects treated), serious skin erosions (13%) appearance of skin inducing and crusts (19%).
Many patients also report pain, itching, burning and irritation in the application area, as well as the feeling of walls. Often you can also verify local bleeding and the appearance of papules.
Treatment for this disease involves the appearance of generalized adverse effects largely superimposable to those recorded in the patients of the previous groups (muscle pain, headache, nausea, lymphadenopathy) to which joint pains and loss of appetite are added, present but with less frequency in the Other groups.
Also for these patients, the most disturbing adverse effects are local ones involving irritation of the zone application of the cream (14% of patients), itching and burning of the injured area (5%). The serious erythema, skin hardening and crust formation (frequency registered around 20%) is extremely common.
In all groups of patients, local skin reactions (erythema, hardening etc.) are probably in relation to an extension of the pharmacological effects of Imequimod Crema. Furthermore, with great frequency in all groups of patients, local superinfections associated with the appearance of pustules can be verified, as well as skin hypopigmentation or localized hyperpigmentation.
IMIQUIMOD is contraindicated in subjects with hypersensitivity known to the active ingredient or to any of the excipients used in the pharmaceutical form.
- External genitalia effects
The cream is applied 3 times a week, before bedtime and must be left to act on the skin for about 6-10 hours. Treatment must continue until the lesions disappeared, for a maximum period of 16 weeks.
- Superficial basocellular carcinomas
The cream is applied 5 times a week, before bedtime and must remain on the skin for about 8 hours. The treatment should continue for a period of 6 weeks.
The cream must be applied before bedtime, 3 times a week and left to act on the skin for about 8 hours. The amount of cream to be used must be sufficient to cover the area affected by the lesions. The treatment should continue for a period of 4 weeks.
Once the application in the following month is suspended, the healing has been evaluated. In case of need, the persistence of signs of attinical keratosis in the area already treated, the treatment can be resumed for an additional 4 weeks.
Experimental studies performed on animals have not highlighted direct or indirect harmful effects on the development of the embryo or fetus, or on post-natal development. At present there are no adequate and controlled studies concerning the use of imiquimod in pregnant women.
The Food and Drug Administration has inserted IMIQUIMOD in class C for use in pregnancy. In this class, drugs whose animal studies have detected harmful effects on the fetus, teratogenic, lethal or other effect are inserted, and for which no controlled studies are available in women or drugs for which no studies are available on man or on the animal. [twelfth] [13]
There are no adequate clinical studies on the possible interactions between imiquimod and other drugs.
From the theoretical point of view, interactions with systemic drugs should be somewhat limited in consideration of the low skin absorption of IMIQUIMOD applied as a cream.
- ^ H. HEMMI, T. KAISHO; O. TAKEUCHI; S. SATO; H. SANJO; K. HOSHINO; T. HORIUCHI; H. TOMIZAWA; K. TAKEDA; S. AKIRA, Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. , in Nat Immunol , vol. 3, n. 2, February 2002, pp. 196-200, doi: 10.1038/ni758 , PMID 11812998 .
- ^ H. Tran, G. Moreno; S. Shumack, Imiquimod as a dermatological therapy. , in Expert Opin Pharmacother , vol. 5, n. 2, February 2004, pp. 427-38, doi: 10.1517/14656566.5.2.427 , PMID 14996638 .
- ^ D. Bilu, DN. Sau, Imiquimod: modes of action. , in Br J Dermatol , 149 Suppl 66, November 2003, pp. 5-8, PMID 14616337 .
- ^ a b Rl. Miller, Jf. Grain; Ml. Owens; Hb. Slade; Ma. Tomai, Imiquimod applied topically: a novel immune response modifier and new class of drug. , in Int J Immunopharmacol , vol. 21, n. 1, January 1999, pp. 1-14, PMID 10411278 .
- ^ IS. Zagon, RN. Donahue; M. Rogosnitzky; PJ. McLaughlin, Imiquimod upregulates the opioid growth factor receptor to inhibit cell proliferation independent of immune function. , in Exp Biol Med (Maywood) , vol. 233, n. 8, Ago 2008, pp. 968-79, DOI: 10.3181/0802-RM-58 , PMID 18480416 .
- ^ PJ. McLaughlin, M. Rogosnitzky; IS. Zagon, Inhibition of DNA synthesis in mouse epidermis by topical imiquimod is dependent on opioid receptors. , in Exp Biol Med (Maywood) , vol. 235, n. 11, November 2010, pp. 1292-9, doi: 10.1258/ebm.2010.010203 , PMID 20975079 .
- ^ S. of Egmond, C. Hoedemaker; R. Sinclair, Successful treatment of perianal Bowen’s disease with imiquimod. , in Int J Dermatol , vol. 46, n. 3, March 2007, pp. 318-9, doi: 10.1111/j.1365-4632.2007.03200.x , PMID 17343595 .
- ^ M. van Seters, M. van Beurden; Fj. Ten Kate; I. Beckmann; Pc. Ewing; MJ. Eijkemans; MJ. Kagie; CJ. Meijer; NK. Aaronson; A. Kleinjan; C. Heijmans antonissen, Treatment of vulvar intraepithelial neoplasia with topical imiquimod. , in N Engl j with , vol. 358, n. 14, April 2008, pp. 1465-73, doi: 10.1056/nejmoa072685 , PMID 18385498 .
- ^ HW. Buck, KJ. Guth, Treatment of vaginal intraepithelial neoplasia (primarily low grade) with imiquimod 5% cream. , in J Low Genit Tract Dis , vol. 7, n. 4, Ott 2003, pp. 290-3, pmid 17051086 .
- ^ JQ. Of red, The use of topical imiquimod for the treatment of actinic keratosis: a status report. , in Skin , vol. 76, n. 4, Ott 2005, pp. 241-8, pmid 16315560 .
- ^ ForUsDoc-Photo gallery: Imiquimod Therapy, Aldara, actinic keratosis . are Forusdocs.com . URL consulted on November 2, 2013 .
- ^ Onyeka Otugo, Olabode Ogundare, Christopher Vaughan, Emmanuel Fadiran, Leyla Sahin, Consistency of Pregnancy Labeling Across Different Therapeutic Classes ( PDF ), are FDA.GOV , Food and Drug Administration – Office of Women’s Health, 1979. URL consulted on November 3, 2013 .
- ^ R. Sannerstedt, P. Lundborg; Br. Danielsson; I. Kihlström; G. Alván; B. Pram; E. Ridley, Drugs during pregnancy: an issue of risk classification and information to prescribers. , in Drug Saf , vol. 14, n. 2, February 1996, pp. 69-77, PMID 8852521 .
Recent Comments