Mycorhize – Wikipedia
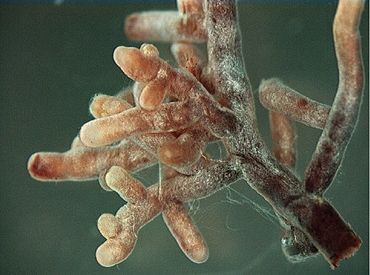


A mycorhize ( myco- coming from the ancient Greek: fungus , mukês , “Mushroom” and Rhiza , “Racine”, a term introduced in 1885 by the botanist Albert Bernhard Frank) is the result of the symbiotic association, called mycorhization , between mushrooms and the roots of the plants. Mycorhize is a major component of the edaphon and the Rhizosphere.
In this generally non -specific association, the spores of a fungus Mycorhicia or mycorrhizogenic (Greek Myco , Rhiza And genus , “Generate”, literally which gives birth to a mycorrhize) are disseminated by the wind (anemochia), the rain (hydrochoria), or by the excrement of animals (endozooochoria), germinate, and give the hyphae of mycelium which colonize the roots of a plant. These radicular hyphae are distinguished from extraradicular hyphae [ 2 ] which also develop outside the root over several centimeters, exploring the surrounding soil of the root system of the host plant. What is commonly called mushroom, which is picked with your foot and hat, is only the “fruiting” of mycelium, sporophore, where sexual reproduction takes place. Hyphles are presented as fine filaments, capable of exploring a very large volume of soil (a thousand meters of mycelial filaments for a meter of root).
The mycorrhizal relationship is of symbiotic type, but an imbalance in the relationship can be induced by a weakness of one of the two partners, the association can then slide along the Mutualism-Parasitis continuum [ 3 ] , [ 4 ] . The fungus can also help recycle the necromasse of its host, for the benefit of their two descendants.
While the exploration surface exploited by the plant is only multiplied by 10 by absorbent hairs, active only during the germination period [ 5 ] , it is multiplied by 10,000 thanks to mycorrhizae (filaments of a diameter of one hundredth of a millimeter) which have an energy cost of implementation a hundred times lower than the roots [ 6 ] . The absorbent hair remains functional in a few groups of adult plants (less than 10%) non -mycorrhized which have lost secondarily the fungal association, more than 90% being colonized by these hyphae of the symbiote fungus which ensure the essentials of the absorption of ‘water and nutrients, and multiply by ten the growth of the plant [ 7 ] . The estimates suggest that there are nearly 50,000 fungal species that form mycorrhizal associations with 250,000 plant species, 80% of these associations corresponding to endomycorrhizaes [ 8 ] . It seems that non -mycorrhized plants of pioneering environments (absence of mushrooms), humid and/or rich, in which the hydromoineral supply does not require mycorrhize, “needed to compensate for the absence of the fungal auxiliary by developing root structures that imitate mycelial filaments and their great efficiency to colonize a large volume of soil [ 9 ] ».
Current research shows a broader association in the mycorrhizae level, with the concept of mycorrhizal microbiome, called rhizomicrobiome, which also involves bacteria [ ten ] , [ 11 ] .


The original symbiote (a glomeromycet it seems) would have appeared about 500 million years ago [ 13 ] At the Paleozoic, probably at the same time as the first terrestrial plants. Fossils from the Rhynie flora (e.g. Aglaophyton (in) , Divide (in) ), aged approximately 400 million years, have morphologically identical mycorrhizae to glomers, associated with rhizomes [ 14 ] . This suggests that mycorrhizae were the instrument of an accelerated colonization of the emerged lands, by their ability to extract water and minerals from the soil, ensuring the first plants their mineral nutrition when they did not yet have roots (Lichens, Bryophytes [ 15 ] In watercourses, terrestrial ferns near the water/firm land limit, such as the edges of fresh or healthy waters) [ 16 ] .


These first associations were also able to allow the constitution of resistant soil better for weathering, better storing water, while improving the resistance of plants to water and cold stress [ 18 ] , [ 19 ] or lack of nitrogen (in spruce for example [ 20 ] ), their winding and runoff, as well as their resistance to too strong light intensities, as is the case of mycophycobiontes of certain foreshore algae (these symbiosis, with mainly ascomycètes, seem to have appeared secondarily).
Mycorrhizal associations (shrubs and ectomycorrhiziennes) also exist in cold ecosystems (average temperature less than 15 ° C), where they are active all or part of the year (depending on the temperature of the soil), involved in certain adaptations of resistance to Gel and promote the acquisition of low -temperature nutrients in mycorrhizal fungi [ 21 ] . They undoubtedly contribute to better survival of the fungus in frozen soils in winter [ 22 ] where microchampignons undergo this additional selection factor [ 23 ] . These cold symbiosis also benefits grasses such as barley (hordeum) which thus improves its access to phosphorus of the ground [ 24 ]
These symbiosis correspond to a “Macroevolutive jump” (leap in evolution) since the functions of one of the partners are associated with the functions of the other, with multiple functional effects [ 25 ] . Indeed, we go from algae and mushroom to that of terrestrial mycorrhizal plant (which goes against Darwinian gradualism).
Currently, 85% of archegoniates, as well as liver, are endomycorrhized by glomers. This supposes that the symbiosis with the glomers is the oldest among the archegoniates and that it would have allowed the impressive radiation of the latter (diversity, lignification …). 80% of vascular plants are colonized by endomycorrhizal fungi while ectomycorrhizaes are often associated with forestry woody [ 26 ] .
The other families of gloméromycetes (Acaulosporaceae and Gigaspora) later appeared around -250/-230 million years. They have superior capacities for the exploitation of mineral resources of the soil.
The ectomycorhiza, on the other hand, may have appeared in the Cretaceous but the oldest known fossils date only from Eocene. They allowed the colonization of soil previously unfavorable. It was also at this time that nitrogen fixing symbiosis appeared [ 27 ] . The symbiosis ectomycorhiziennes has appeared many times independently in different clades of mushrooms (80 times in glomeromycetes, ascomycètes and basidiomycetes) and spermaphytes (12 times in gymnosperms and angiosperms) [ 28 ] . This frequency shows the evolutionary success of these associations from the ancestors of ectomycorrhizal fungi who lived from a deadly organic matter of the soil in a saprophytic lifestyle [ 29 ] . The comparative study of Ectomycorhiziens and current saprophycorhizal fungi shows that mycorrhizal saprophytes have lost many exoenzymes that ensure the autonomous carbon nutrition of saprophytes, because they are fed sugars by the host plant, but have kept enzymes (peroxidases, laccases , fungal phenol-oxidases such as tyrosinases) exploiting the nitrogenous and phosphate resources of organic residues (lignin, phenolic compounds, cellulose) necessary for the proper functioning of the plant [ 30 ] .
Certain more recent mycorrhizal symbiosis plants (from an evolutionary point of view), may also contract an association with mycorrhizaes with Arbuscles. It therefore seems that there have been evolving innovations in this type of symbiosis. These innovations undoubtedly explain the limited number of plants capable of contracting them.
The appearance of ectomycorhiza has been correlated twice to evolving plants:
- at the Cretaceous (appearance of pinaceous and rosidaus);
- During the Eocene-Oligocene transition (appearance of “current” forests of the northern hemisphere). This appearance would be an adaptation to temperate regions where, during the cold or dry season, the mineralization of organic matter and the alteration of rocks are slowed down. However, some Ectomycorrhiziennes species have greater capacities to alter rocks by their hyphae and others have enzymatic equipment allowing them to extract nitrogen residues or phosphate from organic matter from the soil.
More recently [When ?] Still other forms of endomycorrhiza and pelot ectomycorrhizae have appeared, especially in Eicales, with the phenomenon of mycoheterotrophy: their fungal symbiotes have even stronger saprophyte capacities allowing the plant to directly reinge organic carbon by the Biases of the fungus, in the soils where the mineralization activity is very low (moors, high mountains, cold ecosystems …): the symbiosis allows a coupling of trophic levels.
Several groups of plants (less than 10%) are not mycorrhized. They have lost secondary the association and illustrate the phenomenon of neotenia. These are mainly pioneering (absence of mushrooms) or rich plants. For example Bryophytes adapted to hostile environments, where they can dry out and where a fungus would survive badly. They have acquired anatomical structures allowing them to resist the desiccation but, correlatively, their size remains small. These are also angiosperms (brassicaceae, chénopodiaceae, polygonaceae, proteced, caricaceous, etc.) that have most often adapted to rich environments, in which hydromineral supply does not require mycorrhize [ 7 ] .

Mycorrhizae are at the origin of the most complex ecosystems, and in particular in forests and in particular tropical forests which live and often evolve on ungrateful and sometimes not very fertile soils. Their myceliums form interconnected mycelial networks which influence the functioning of ecosystems (biogeochemical cycles, composition of plant communities, carbonated food food during their development, modification of competition, etc.) by allowing or increasing significant flows of organic carbon and minerals ( nitrogen, phosphorus, water, etc.) via soil (on average 30 to 40% of minerals captured by the margins of the mycelial network are returned to the root, the latter bringing 4 to 40% of photosynthesized carbohydrates in mushroom [ thirty first ] , [ 32 ] , [ 33 ] ). They are one of the most dynamic elements of the mycorrhizal symbiosis and play an essential role in the functioning and structuring of plant communities. These transfers are so effective, that they call into question the concept of competition by competition for nutrients between the plants of an ecosystem, in particular for the capture of phosphates by the roots (they allow to do without fertilizers phosphate), For toxic calcium resistance [ 34 ] (some calcicole plants tolerate calcium thanks to mycelial hyphae actively rejecting outside the CA ions 2+ or by immobilizing them in the form of calcium oxalate crystals which precipitate in vacuoles, idioblasts, or on the walls of hyphae) [ 35 ] or for drought resistance. However, they are still little exploited in horticulture, agriculture and forestry, or for the depollution of certain polluted soils.
Some groups of mushrooms are probably key species or even “engineering species” that influence the main ecological processes of the soil. They are considered by socks as essential elements of the diversity of communities, which is a factor of stability and ecological balance [ 36 ] . Many key groups found in the soil (bachelor and mycorrhizal fungi in particular) can connect to plants (at least 90% of families of land plants are affected) via mycorrhizal associations with shrubs and play essential synergies for survival and plant productivity, helping to form an essentially underground ecological network, which certain biologists have appointed the wood-wide web (referring to “World Wide Web [ 36 ] »). It is Canadian researcher Suzanne Simard who is the first to highlight this network in 1997 with the mycorrhizal carbon transfer between trees in natural conditions [ 37 ] .
Most mycorrhizal mushrooms are suspected of having several hosts or even a wide range of hosts (each plant being commonly associated with several dozen different mycorrhizal mushrooms) [ thirty first ] , which seems to be confirmed in natural circles, but studies done on cultivated arable soils show, however, that the diversity in mycorrhizal fungi is there “Extremely low compared to forest soils” [ 36 ] .
The colonization of root systems, the “mycorrhizogenic” potential of the soil and the “mycorrhizal dependence” of the plants are inversely correlated with the content of the soil solution in phosphate ions; In addition, this result is not linked to a form of phosphate fertilizer, whether organic or mineral, since plants only absorb ions in solution. The enrichment of this solution becomes directly responsible for the fact that the well -fed plant no longer promotes the development of mycorrhizae. In certain situations, the levels of phosphorus affected become incompatible with the installation of mycorrhizae [ 38 ] .
Mycorrhizae also interact with various soil bacteria (including Pseudomonas ) which can be pathogenic (mycorrhize protects the plant against its pathogens, for example by emitting antibiotics [ 39 ] ), but which are also called “auxiliary bacteria to mycorrhization” (in English MHB: Mycorrhizal Helper Bacteria) as they play an important role.
Mycorrhizae also interact with other mycorrhizae and other mushrooms and with certain predators and plants of plants:
- The attack of plants by herbivores causes a rapid modification of mycorrhizal communities (the species requiring the least carbon are favored) but the nature of the Mycorrhizal population also modifies (positively or negatively) the defense capacities of plants;
- The mycorrhizal communities respond (by modification of the specific abundances) to environmental changes depending on whether they are more or less favorable to the host or symbiote and bacterial communities are modified by the variation of exudates between mycorrhized roots and not mycorrhized;
- The diversity of mycorrhizal mushrooms with ground arbuscles controls the composition of plant communities by a direct effect of the fungus on the selective value of individuals they colonize (beneficial, neutral, negative or even suppressive effect). This phenomenon is linked to the preference of existing host for each mushroom. This preference would reside in the adequacy between ecological functions exercised by the fungus and needs of the host plant.
Many experiences of Controlled mycorrhization have shown that natural or artificial regeneration benefited from the presence or inoculation of symbiotic mushrooms adapted to plants and context (mushroom products and effective microorganisms containing strain cocktails). Conversely, truffle farming could only be exported to New Zealand with mycorrhized host plants. Likewise European Christmas trees only pushed it with their symbiotes. Some symbiotes are spectacular: it is a 60% increase in the total volume of Pseudotsuga menziesii which has been allowed in 10 years on afforestation from ectomycorrhized plants in nursery with a strain Laccaria bicolor called S238N, compared to uninoculated trees [ 40 ] .
Precautions : The risk exists of competition with local species, even genetic pollution. Several follow -ups have shown that plants inoculated in nurseries have generally lost this symbiote in favor of others from the local soil, but it may not always be the case [ 41 ] .
Unlike a widespread idea, the mycorrhizal relationship is not exclusively symbiotic because the transfers of fungus substances to the plant do not always increase the selective value (which is the criterion of definition of symbiosis).
The benefits, which vary highly depending on the genotype of partners and the environment are difficult to assess, particularly for perennial plants. They could benefit from symbiosis only for short periods at different times of their lives. There is in fact a continuum of relations between partners, from symbiosis to parasitism through saprotrophy (the nature of the relationship is descriptible according to the cost/benefit ratio).
For example, Tricholoma Matsutake is symbiotic, parasitic or saprophyte according to its stage of development and the conditions of the environment; Orchid mycorrhizae are parasitic of woody and bird’s nest neottie are parasitic of their mycorrhizae.
In fact, mycorrhization is continuously unstable due to conflicts of interest between partners and the selection pressure that pushes each partner to be as parasitic as possible, which explains the many possible interactions: exchanges of nutrients (the plant provides sugars and lipids, fungal myceliums provide water and mineral salts that it draws from the ground, sometimes to several tens of centimeters of the root [ 42 ] ), growth factors (hormones, vitamins), allomones providing protection [ 43 ] . Too much parasitism can lead to the rupture of the association. Over time, the “symbiosis” would have appeared and would have been broken several times: the association is reversible. Saprophytes mushrooms could thus be symbiotes who have lost their host.
Thus, in certain Mycorrhiziennes associations, one of the partners seems to exploit the other (either by nature, or according to the conditions). Here we mean by “operator” an individual who obtains a profit (increase in selective value) without reciprocity.
We can cite as an example mycoheterotrophic plants (more than 200 known species in orchidaceae, gentianaceae, buranniacae …), plants with zero or reduced photosynthetic capacities which obtain their carbon from their Mycorhizian partner, he himself obtaining ‘A chlorophyll partner with the mycelial network.
The exploitation of one of the partners of the symbiosis by the other supposes compensations for the exploited individual:
- Genetic uniformity: Symbiosis helps maintain the genetic uniformity of the species which is sometimes more interesting that is more interesting than variability (this is the case with vesicles and shrubscules and ericoids);
- Vertical transmission: The selective value of the exploited is linked to that of its partner. This is for example the case for glomers who have lost any sexual reproduction for 400 million years and are dependent on the host for their survival;
- Neighborhood interaction: when the density of exploiter is large, the exploited can take refuge on the least operating hosts and reduce the selective value of more demanding hosts.
- commensalism;
- Promisescuity: ability to associate with several partners (case of mycoheterotrophic).
Compensation mechanisms could be important in stabilizing symbiosis. In general, symbiosis is characterized by a decrease in genetic drift and the specification rate (see “Red King” Effet [ 44 ] ) But this is not always the case in mycorrhization: the more the association is exploiting the more it is specific because the exploited develops resistances which must be bypassed by the exploiter (theory of the red queen). In this case, the selective pressure on the host would have led to the appearance of lignin and favored the growth of vascular tissue.
Finally, logic and many clues suggest a parasitic origin of mycorrhizae:
- Almost half of the genes whose expression is modified by mycorrhization in the same way during an attack of fungal parasites (as an example, the elicitation of the chitinases);
- During evolution, there was counter-selection of defense genes and increased symbiosis genes. The stimulation of the basal defenses (non -specific) of the plant by mycorrhizae would therefore be a vestige of the old parasitic relationship between the partners.
- Many other examples of symbiosis or lasting interactions seem to originally have parasitism.


There are two main types of mycorrhizae, defined by the physioanatomical relationships between the two partners [ 27 ] : Endomycorhiza and ectomycorhizaes. Ericoids and those associated with orchids are also studied for their ecological services, but have a more limited economic interest.
A global cartography of forest symbiosis [ 45 ] shows the evolutionary success of ectomycorrhized trees. On the three most common types of symbiosis (endomycorrhizae with abundant shrubs in warm and humid tropical climates, ectomycorhizaes in cold climates, and nitrogen fixing bacteria in arid and hot climates), ectomycorhizae represent only 2% of plant species, but are 60% of all the trees on the planet [ forty six ] , [ 47 ] .
Endomycorhizes [ modifier | Modifier and code ]
Endomycorhiza (or internal mycorrhizae) are the most widespread form. These are mycorrhizae who penetrate inside the roots to better associate with it. There are several types of endomycorrhizae:
Arbuscles endomycorrhizae [ modifier | Modifier and code ]



Endomycorrhizaes with shrubs or shrubs (AM) are the most common case. These mycorrhizal fungi colonize around 80% of terrestrial vascular plants, that is to say more than 400,000 species. However, there are less than 200 species of endomycorrhizal fungi. These fungi are therefore not very specific in their symbiosis relationships. Being little specific, each species must have a great adaptability potential and a large genetic diversity in order to allow it to adapt to the different environmental conditions it must face.
They are associated with herbaceous and woody plants. These armbuscular endomycorrhizaes, also called vesicles and shrubs (or vesiculum-arbuscular mycorrhizae), take their name from intercellular vesicles (often oil drops, reserve storage for the fungus) and “intracellular” structures reminding a small tree . If they cross the wall well, they do not penetrate the plasma membrane of the plant cell, however, contenting themselves with causing an invagination of its membrane. This has the effect of increasing the contact surface between the hyphery and the plant cell and thus facilitating the exchange of metabolites between the two partners.
Arbuscular endomycorrhizae are only formed by fungi from the glomeromycetes division that has lost sexual reproduction. The hyphae extend into the cortical parenchyma of the root, forming vesicles containing reserves, and branched structures, the shrubs. They therefore reproduce only asexually. However, hyphae of different individuals can merge, which makes possible a genetic exchange and a form of parasexuality
They are also unique from a genetic point of view since their spores have several genetically different nuclei [ 48 ] .
Intracellular platform endomycorrhizae [ modifier | Modifier and code ]
In endomycorrhiza with intracellular platforms, hyphae form piles in cortical cells. They involve Basidiomycetes, in association with the orchidaceae which increased their dependence on the symbiosis, by becoming mycoheterotrophies which exploit their mycorrhizal mushrooms as a carbon source [ 49 ] . The hyphae penetrate through the wall of the cells inside the cells of the root cortex by pushing the plasma membrane. The hyphae wall is therefore in contact with the plasma membrane of the root cell, without crossing it. The contact surface can be increased by the formation of ramifications (or shrubs). The roots are not deformed.
Enricoid endomycorrhiza [ modifier | Modifier and code ]
In ericoid endomycorrhiza: hyphae form platoons in transient roots of low diameter. They involve ascomycetes or basidomycetes (in symbiosis with Ericales).
Ectendomycorhizes [ modifier | Modifier and code ]
In ectendomycorhizaes, also called mycorrhizae ectendotrophes or arbutoid type mycorrhizae: the fungus forms intracellular platoons like endomycorrhizaes, and a coat around the root like ectomycorhizaes. This is the case with pyroles who exploit, like orchidaceae, their mycorrhizal fungi as a carbon source [ 50 ] . There are also monotropoïdes echerscorrhizae, in non -chlorophyll Ericals. The hyphae form platoons in the superficial cells of the root.
In this symbiosis, the dense and extended network of mycorrhizal fungi hyphs helps the plant explore an increased volume of soil and to access places inaccessible for roots. The fungus allows the plant to improve its nutrition by mainly bringing water, phosphorus and nitrogen. Incidentally, the colonization of the roots by mycorrhizal fungi helps protect them against attacks by pathogenic organisms. Several studies have shown that without association with a symbiotic fungus, the plant will grow more slowly (if not at all, as in the case of black pine on limestone soil) and will be much more likely to be the victim of an infection. In return, the fungus benefits from the photosynthesis of the plant in the form of organic matter rich in energy (sugars) essential to its survival. The quality of humus is improved, for the benefit of other species and the maintenance or constitution and improvement of the soil.
Some plants from the gymnosperm family such as podocarpaceae, araucariaceae and phyllocladaceae, are known to present root bulges invaded by endomycorrhizal mushrooms, called myconodules or pseudonodules .
Ectomycorhizes [ modifier | Modifier and code ]

Ectomycorrhiza (or external mycorrhizae) concern only 5% of vascular plants, mostly trees in temperate and boreal forests (such as fagaceae, pinaceae or concrete) and mushrooms of the ascomycetes, basidiomycetes or zygomycetes division. These mycorrhizae do not penetrate through cell walls inside the cells of the plant, but simply surround the roots, forming a mycelium coat and a network between the walls of the cells of the root.
The fungus is first associated with fine growth roots with determined growth, devoid of absorbent hair. Then, it envelops the root of a hyphae coat, the mycorrhizal sleeve. Other hyphae grow between cells in the external part of the cortical parenchyma, thus forming the symbiotic interface or ” Hartig network ». The symbiosis modifies the physiognomy of the mycorrhized root: it is swelling, stops growing and can be branched out. The cap and the apical meristem are then reduced.
Hundreds of different mushrooms can be associated with the same species. Beech, for example, has a “record” with more than 200 Mychoriziennes associations.


Inductors of the host plant [ modifier | Modifier and code ]
The growth of fungal mycelium towards the roots of the host plant is triggered by the perception of a signal emitted permanently by the roots in the form of molecules specific to the plant such as strigolactones, and especially flavonoids [ 51 ] .
Myc signaling track [ modifier | Modifier and code ]
The signals issued by the fungus which allow it to be recognized by the plant are poorly known. By analogy with NOD factors, these “myc factors” factors are called. NOD factor -type molecules produced by shrub fungi and having an effect on the host plant have recently been highlighted [ 52 ] , but their role in symbiotic signaling remains to be determined.
The signaling route activated by the MYC factors presents common steps with the NOD route present in legumes nodulated by rhizobia [ 53 ] and in Actinorhizian plants [ 54 ] . Myc factors receptors are not known, but proteins intervening after such as Symrk, Castor, Pollux, Nup, Cyclops are necessary for Rhizobian and Hycorrhizian Symbiosis. As with the Rhizobian symbiosis, contact with an shrub fungus induces calcium oscillations.
Arbuscular endomycorrhizae having appeared before nitrogen fixing endosymbiosis, researchers hypothesize that the transduction route of the Mycorrhizal signal has been recruited by nitrogen fixing bacteria.
An optimal mycorrhizal procession would act like a “fertilizer” without its drawbacks, and free of charge, by pesticides, fungicides and amendments. After first tests in Australia in the 1920s, companies developed inoculation strains adapted to several commercial species (monosouche or multi -purchase inoculums), but they should be also adapted to the context of the location. Several researchers believe that the strains of mushrooms symbiotic genetically best suited to the field, are those which are indigenous [ 55 ] . Private companies around the world have thus developed the production and sale of mycorrhizal inoculants intended for agricultural use (bi -fertilizing, biostimulant) but also ecological (Revégetalization of eroded soils, phytoremediation) [ 56 ] .
Taken into account in the management of forests and cultures [ modifier | Modifier and code ]
In agriculture, the use of mycorrhizal fungi would avoid many problems of denaturing organic soils. Indeed, the addition of pesticides and in particular fungicides can have harmful effects on the quality of the soil. Mushrooms have important roles in maintaining fertile soil. The addition of highly phosphorus chemical compounds would induce impoverishment in quantity and quality of endomycorrhizal fungi, reducing nutrient intake to cultivated plants. In addition, too much phosphorus has a harmful impact on rivers (eutrophication) [ 57 ] . It is essential then to reduce the amount of harmful chemicals and rather opt for the inoculation of natural fungi that would have the same rapid growth advantages, without the disadvantages on the environment.
A study on jujube has shown that controlled mycorrhization was beneficial to its growth and phosphate nutrition. Indeed, the fruit tree absorbed with much more ease the natural phosphorus added in poor soil. He was even dependent on normal growth [ 58 ] . It is therefore not enough, in agriculture, to simply add phosphorus in very large quantities so that the plant grows faster, it is also a question of taking into account the Mycorhizian network [ 59 ] . It must first and foremost be able to absorb effectively. An overdose of nutrients would not change anything and that would only cause phosphorus leaching and surface water pollution.
The action of mycorrhizae does not stop at better nutrition: they also allow the plant to better protect itself from harmful biotic and abiotic factors. Of course, better nutrition involves better health in plants which can then better tolerate environmental stress, especially pathogens.
Mycorrhizae can release antibiotics in the rhizosphere, causing the elimination of all microorganisms, pathogens or not, which could divert part of the exchanges for their benefit [ 60 ] . In addition, the mycorrhizal symbiosis also induces the establishment of defense mechanisms of the plant itself (premunition and immune stimulation). It is an indirect protection that is at the cellular level. In ectomycorrhized plants, rhizoderm cells synthesize tannins stored in vacuoles which constitute premunition in the face of other attacks of microbes and other pathogens. The plant also produces more lignin in the cell walls of endoderm and vascular tissues. It can also induce or remove various defenses linked to phytoalexins, peroxidases, chitinases and several others. In short, the fungus causes considerable changes in defense mechanisms in the plant against various parasites, before infections [ sixty one ] .
Sometimes the association with a fungus causes reshaping of the root system. For example, in strawberry, mycorrhization causes increased protection against the root rot caused by Phytophthora fragariae . The intense ramification of the roots induced by the Mycorrhizal fungus is accompanied by root exudats, and this would have the effect of modifying microflora and direct interactions with parasites [ 62 ] .
Regarding parasitic mycytes, a plant associated with a mycorrhize is also better protected. The hyphae of the symbiotic fungus colonize the roots of the plant and “block” sites of access to the parasitic my drizzle. They also compete with the available nutrients. A mycorrhize also receives advantages to bind to a plant: it receives carbon compounds in exchange for minerals. Carbon being very coveted, a mycorrhize is more advantaged than a free parasitic fungus in the ground. In addition, mycorrhizae can represent up to 80% of the microbial mass of the soil [ 63 ] . They then greatly influence the physical and chemical properties of the environment and can therefore control several microbial interactions of the soil. Sometimes it is in accordance with the mycorrhized plant by providing protection and growth, but sometimes the effects are rather negative [ sixty four ] .
Mycorrhizae can therefore help protect the plant with abiotic stress (for example drought) and biotic in several ways: better nutrition and health, morphological transformation of the roots, induction or suppression of defense mechanisms and acting on the parasite itself, either by competition for resources and infection sites, or by modifying microflora and the increase in the rate of organic matter [ sixty one ] .
It should be noted, however, that these interactions are studied in greenhouse and in controlled environments and that they therefore do not completely reflect all the complexity of a natural environment variable in time and in composition [ sixty one ] .
With all the advantages set out in mycorrhizae, it is therefore just to say that their good use would avoid many environmental concerns because they would act as fertilizers and protective agents. The use of chemical fertilizers, fungicides and pesticides would no longer be as necessary. Indeed, fertilizers decrease the rate of mycorrhization, which has an increased dependence of plants to these inputs. Likewise, the use of pesticides decreases the inoculating power of the soil, hence a decrease in the protective effect of mycorrhizae and an increased demand for pesticide plants. The addition of such inputs installs a vicious circle shape [ 65 ] .
The silvicultural and agricultural activity can disrupt or modify (negatively or positively) the fungal microflora and its ability to mycorrhizer;
- The turnover of the soil (plowing), the complaint by devices damage the ectomycorrhizal community
- The genetic selection and improvement programs of plants adapted to high -level input crop systems (nitrogen fertilizers, phosphate, potassiums), minimize or cancel the training of mycorrhizae. This process may be reinforced by varietal selections which relate to resistance to fungal diseases [ 66 ] , [ sixty seven ] .
- Nitrogen fertilization (which is done more and more in forestry too) decreases the number of carpophores and alters the composition in cash (measured on Picea abies by Peter et al. [ 68 ] , by reducing the number of mycorrhizae [ 69 ] , and by changing mycorrhizal types (Brandbrud, 1995; Karen, 1997).
- The shaved cuts damage the Mycorrhizian community [ 70 ] . Jones believes that the change in composition of ectomycorrhizal communities is more in question than a decrease in the root colonization rate. The soil compaction could also be at stake, the same in the long term as the export of almost all the woody biomass.
- Flooring forest cuts [ 71 ] , [ 72 ] , as well as irrigation [ seventy three ] Increase the number of carpophores produced with effects that are still poorly understood on the composition and biodiversity of the fungal community.
- Of course the use of fungicide affects this community. The presence of fungicides in meteoric waters (rains, mists, dew, snow, etc.) is proven, but its impacts on the mushrooms remain poorly understood.
Biochemical and biological changes, as well as microclimatic induced by large, shaved cuts, (or even harvests in the case of agriculture) could have underestimated impacts, linked to direct impacts on the ground, on water , but also to the loss of fungal inoculum (Even if the roots remain in the ground, the “big-bois” and large “dead wood” become rare or absent, and the remanent are often gathered).
The term mycorrhize (from Greek Myco , “Mushroom” and Rhiza , “Racine”) was introduced in 1885 by botanist Albert Bernhard Frank [ 74 ] . Engaged by the King of Prussia Guillaume I is To develop truffle production methods by studying their propagation mode, A.B.FRANK observes that these fungi combine the royalties of trees and proposes that this association is a symbiotic and non -parasitic relationship. Frank’s evolutionary ecology theory, contradicting the belief that truffles and other fungi lead to plant diseases and rot, is strongly disputed by his colleagues [ 75 ] . This theory returns to the front of the stage with the work [ 76 ] The Jack Harley [ 77 ] , [ 78 ] considered the “Pope of mycorrhizae” For his studies since the 1950s [ 79 ] , Barbara Mosse a 1962 [ 80 ] , J.M. Phillips and D.S. Hayman in 1970 [ 81 ] , M. Gardes and T.D. Bruns in 1993 [ 82 ] .
Research is in full development at the start of XXI It is century thanks to the new means of molecular biology and genetics, to the point of giving the name to a scientific discipline, the mycorhizologie , studied by mycorhizologues [ 83 ] . From 2008 to 2016, more than 10,000 new scientific contributions concerned the mycorrhizae [ 38 ] .
- Legend: a = root cortex, b = root epidermis, c = armbuscle, d = vesicle, e = hyphery, f = absorbent hair, g = cell nuclei.
- Also called extramatrial hyphae.
- Margaret L. Ronsheim, The Effect of Mycorrhizae on Plant Growth and Reproduction Varies with Soil Phosphorus and Developmental Stage ; The American Midland Naturalist (published by the “University of Notre Dame”) 167 (1); Pages 28 to 39. Jan 2012 DOI: https://dx.doi.org/10.1674/0003-0031-167.1.28 ( Résumé )
- Some mycorrhizal mushrooms become parasitic when their carbon cost surpasses their hydro-mineral contribution. See (in) Melanie D. Jones, Sally E. Smith, ‘ Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? » , Canadian Journal of Botany , vol. 82, n O 8, , p. 1089-1109 (DOI 10.1139/B04-110 ) .
- The lessons are often based on plant observations in germination, which masks the fact that most of the absorbent hairs of adult plants are not functional. This vision has long prevailed in researchers in plant biology who have used as an experimental model exceptionally non -mycorrhizal species, like Arabette ( Arabidopsis thaliana , from the cabbage family).
- As a mushroom hyphery has a radius ten times smaller than a root hair, its surface/volume ratio is a hundred times larger, hence the 1,000/10 ratio. “In a meadow, each meter of root corresponds to 10 kilometers of hyphae. Each cm 3 of soil contains between 100 and 1,000 m of hyphae, the surface of which is, by proxy, the indirect contact between the plant and the soil. Under 1 m 2 of soil, the surface of the hyphae represents approximately 100 m 2 » . Cf Marc-André Selosse, Never alone. These microbes which build plants, animals and civilizations , Acts Sud Nature, , p. 58 .
- Marc-André Selosse, Never alone. These microbes which build plants, animals and civilizations , Editions Actes Sud, , p. 171
- (in) van der Heijden MG1, Martin FM, Selosse Ma, Sanders Ir, ‘ Mycorrhizal ecology and evolution: the past, the present, and the future » , New Phytol , vol. 205, n O 4, , p. 1406-1423 (DOI 10.1111/NPH.13288 ) .
- Jean Garbaye, Mycorrhizal symbiosis Editions are , p. 121 .
- (in) Jacqueline M. Chaparro, Dayakar V. Badri, Matthew G. Bakker, Akifumi Sugiyama, Daniel K. Manter, Jorge M. Vivanco, ‘ Root Exudation of Phytochemicals in Arabidopsis Follows Specific Patterns That Are Developmentally Programmed and Correlate with Soil Microbial Functions » , PLOS ONE , vol. 8, n O 2, (DOI 10.1371/journal.pone.0055731 ) .
- (in) Alessandro Desiò, Alessandra Salvioli, Eddy L Ngonkeu, Stephen J Mondo, Sara Epis, Antonella Faccio, Andres Kaech, Teresa and Pawlowska & Paola Bonfante, ‘ Detection of a novel intracellular microbiome hosted in arbuscular mycorrhizal fungi » , The ism journal , vol. 8, , p. 257–270 (DOI 10.1038/ismej.2013.151 ) .
- (in) Frida A. A. Feijen, Rutger A. Vos, Jorinde Nuytinck & Vincent S. F. T. Merckx, ‘ Evolutionary dynamics of mycorrhizal symbiosis in land plant diversification » , Scientific Reports , vol. 8, n O 1, (DOI 10.1038/s41598-018-28920-x ) .
- Mycorrhize, the symbiosis that has made earthly life , For science n O 494, December 2018.
- Bernard Boullard and Y. Lemoigne, ” Endophyte fungi of “rhynia gwynne-vaughanii” K. and L. morphological study and deductions on their biology », Botanist , vol. 54, n you 1-6, , p. 49-89 .
- Hepatic and anthocerotic but not the bryophytes s.s. (foams, sphangues). See (in) B. Wang & y.-l. qiu, ‘ Phylogenetic distribution and evolution of mycorrhizas in land plants » , Mycorrhiza , vol. 16, n O 5, , p. 299–363 (DOI 10.1007/s00572-005-0033-6 ) .
- Jean-Christophe Guéguen, David Garon, Biodiversity and evolution of the fungal world , EDP Sciences, , p. 96
- “The general shape and the color of the ectomycorhiza vary according to the species of the infective fungus and for the same fungus the form can vary according to the infested tree” . Cf Jacques Delmas, Mushrooms and their culture , Flammarion, , p. 51 .
- Addy HD, Miller MH, Peterson RL. 1997. Infectivity of the propagules associated with extraradical mycelia of two AM fungi following winter freezing . New Phytol. 135: 745-753
- Addy HD, Boswell EP, Koide RT. 1998. Low temperature acclimation and freezing resistance of extraradical VA mycorrhizal hyphae . Mycol. Res. 102: 582-586
- Alexander IJ, Fairley RI. 1983. Effects of N fertilization on populations of fine roots and mycorrhizas in spruce humus . Plant Soil 71: 49-53
- Mark Tibbett, John W.G. Cairney, The cooler side of mycorrhizas: their occurrence and functioning at low temperatures ; Canadian Botanical Review, 2007, 85 (1): 51-62, 10.1139/B06-152; Online: 2007-04-05; ( Summary, in French )
- Addy HD, Schaffer GF, Miller MH, Peterson RL. 1994. Survival of the external mycelium of a VAM fungus in frozen soil over winter. Mycorrhiza 5: 1-5
- Antal Z, Manczinger L, Szakacs G, Szágády RP, Ferenczy L. 2000. Colony growth, in vitro antangonism and secretion of extracellular enzymes in cold-tolerant strains of Trichoderma species . Mycol. Res. 104: 545-549
- Barnon hb, Yshifs all stones it. 19944. Phosphours uptake and growth of Barley as affected by soil temperature and mycorrhizal infection . J. Plant Nutr. 17: 547-552
- Marc-André Selosse, François Le Tacon, ” Symbiotic strategies to conquer the earthly environment by plants », Biol year. , vol. 40, , p. 15-16 ( read online )
- Jean Pelmont, Biodegradations and metabolisms , EDP Sciences, , p. 741
- Francis Martin, “Plants and Mushrooms, associations with reciprocal benefit”, conference at the City of Sciences and Industry, October 26, 2010
- Marc-André Selosse, Never alone. These microbes which build plants, animals and civilizations , Editions Actes Sud, ( read online ) , p. 145
- (in) D.S. Hibbett, L.B. Gilbert, M.J. Donoghue, ‘ Evolutionary instability of ectomycorrhizal symbioses in basidiomycetes » , Nature , vol. 407, , p. 506-508 ( read online )
- (in) Gary D. Bending, David J. Read, ‘ Lignin and soluble phenolic degradation by ectomycorrhizal and ericoid mycorrhizal fungi » , Mycological Reseach , vol. 101, n O 11, , p. 1348-1354 (DOI 10.1017/S0953756297004140 )
- Marc-André Selosse, Franck Richard, Pierre-Emmanuel Courty, ” Plants and mushrooms: the vital alliance », The research , n O 411, , p. 59 .
- (in) P. G. Kennedy, A. D. Izzo, T. D. Bruns, ‘ There is high potential for the formation of common mycorrhizal networks between understorey and canopy trees in a mixed evergreen forest » , Journal of Ecology , vol. 91, n O 6, , p. 1071-1080 (DOI 10.1046/j.1365-2745.2003.00829.x ) .
- (in) H. Bücking, E. Liepold, P. Alogen « The role of the mycorrhizal symbiosis in nutrient uptake of plants and the regulatory mechanisms underlying these transport processes » , dans dhal nk, sahu sc, Plant Science , Intech, (DOI 10,5772/52570 ) , p. 108-132
- Excess calcium disrupts the functioning and arrangement of the molecules of cellular membranes.
- (in) Kermit chromack et al., ‘ The role of oxalic acid and bicarbonate in calcium cycling by fungi and bacteria : some possible implications for soil animals » , Ecological Bulletins , n O 25, , p. 246-252
- T. Helgason, T. J. Daniell, R. Husband, A. H. Fitter & J. P. W. Young, Ploughing up the wood-wide web ? , Nature, Scientific Correspondence Nature 394, 431 (30 July 1998) ; Doi:10.1038/28764 ( Résumé )
- (in) Suzanne Simard, David A Perry, Melanie D. Jones, David D. Myrold, ‘ Net transfer of C between ectomycorrhizal tree species in the field » , Nature , vol. 388, n O 6642, , p. 579-582 (DOI 10.1038/41557 ) .
- Fortin, Planchette et Piché – “Les Mycorhizes, La Nouvelle Revolution Green”, Quae, 2008, p.96, reissued and increased in 2016:
- Francis Hallé, ” Underground leaves? », Alloy , n O 64, , p. 93
- Selosse M.A., Bouchard D., Martin F. & Le Tacon F. 2000. Effect of Laccaria bicolor strains inoculated on Douglas-fir (Pseudotsuga menziesii) several years after nursery inoculation. Canadian Journal of Forest Research 30: 360-371.
- Thesis by André Gagné, molecular study of the procession ectomycorhizien of conifers plantations on forest sites after white cuts, Laval University, 2005
- Marc-André Selosse, Franck Richard, Pierre-Emmanuel Courty, ” Plants and mushrooms: the vital alliance », The research , n O 411, , p. 58 .
- (in) M-A. Selosse, F. Richard, X. He & S. W. Simard, ‘ Mycorrhizal Networks: dangerous links? » , Trends in Ecology and Evolution , vol. 21, n O 11, , p. 621-628 .
- The Red King effect: When the slowest runner wins the coevolutionary race, Carl T. Bergstrom and Michael Lachmann, Proceedings of the National Academy of Sciences
- Global map of forest symbiosis: Ectomycorhized (A) trees, trees with endomycorrhizaes with shrubs (B), and nitrogen fixing bacteria (C) , Tiré de B. S. Steidinger et al., 2019
- (in) B. S. Steidinger, T. W. Crowther, J. Liang, M. E. Van Nuland, G. D. A. Werner, P. B. Reich, G. Nabuurs, S. de-Miguel, M. Zhou, N. Picard, B. Herault, X. Zhao, C. Zhang, D. Routh, K. G. Peay & GFBI consortium, ‘ Climatic controls of decomposition drive the global biogeography of forest-tree symbioses » , Nature , vol. 569, , p. 404–408 (DOI 10.1038/S41586-019-1128-0 ) .
- ‘ A global map of the microbial symbiosis of the trees reveals their key role in the regulation of the climate » , on CIRAD.FR , .
- [first]
- Marc-André Selosse, Jean Jacques Guillaumin (2005), From germination to adulthood: symbiotic mushrooms of orchids, in M. Bournérias & D. Prat (ed.), Orchids of France, Belgium and Luxembourg, p. 34-44. Parthenope
- Marc-André Selosse, ” Are there plants without symbiosis? », Friends of the National Museum of Natural History, quarterly publication , The friends of the Museum, n O 266, , p. 24 .
- Jean Garbaye, Mycorrhizal symbiosis Editions are , p. 66 .
- http://www.wipo.int/pctdb/en/wo.jsp?wo=2010049751
- http://linkinghub.elsevier.com/retrieve/pii/s1937644810810810019
- http://www.pnas.org/content/105/12/4928.full
- Trappe J.M. 1977. Selection of fungi for ectomycorrhizal inoculation nurseries. Annual Review of Phytopathology 15: 203-222. ;
Navratil S. 1988. The State of the Art in Mycorrhizal Research in Alberta and Saskatchewan. In Proceedings of the Canadian Workshop on Mycorrhizae in Forestry, May 1-4, 1988. M. Lalonde & Y. Piché (ED). Research center in forest biology, Faculty of Forestry and Geodesy, Laval University, Ste-Foy, Qc .;
Perry P.D., Molina R. & Amaranthus M.P. 1987. Mycorrhizae, mycorrhirospheres and reforestation. Canadian Journal of Forest Research 17: 929-940. - (in) Gianazzi G, Gollotte A, Binet M-N, Van Tuinen D, Red WD, ‘ Agroecology: the key role of arbuscular mycorrhizas in ecosystem services » , Mycorrhiza , vol. 20, n O 8, , p. 519–530 (DOI 10.1007/s00572-010-0333-3 ) .
- R. Chaussod, ‘ The biological quality of soils, assessments and implications » (consulted the ) .
- Amadou Beby, Tiby, Labobinn, Ausmannte, Bustle, Kuusdaa Sacko, Koouda Sidigui, and Babadéou, the Waldé, Na Bamadou Controlled mycorrhization and phosphate fertilization: applications to the domestication of jujube », Fruits , vol. 56, n O 04, , p. 261-269 ( read online )
- ‘ Mycorrhizal networks, soils and agriculture: a story to invent! » , on blog.defi-efologique.com , (consulted the )
- Azcon-Aguilar, C. et J.M. Barea, « Arbuscular mycorrhizas and biological control of soil-borne plant pathogens – an overview of the mechanisms involved », Mycorrhiza , vol. 6, n O 6, , p. 457-464 ( read online )
- Yolande Dalpé, ” Mycorrhizae: a plant protection tool but not a panacea », Phytoprotection , vol. eighty six, n O 1, , p. 53-59 ( read online )
- Norman, J.R. et Hooker, J.E., « Sporulation of Phytophthora fragariae shows greater stimulation by exudates of non-mycorrhizal than by mycorrhizal strawberry roots », Mycology , n O 104 : 1069-1073.DOI:10.1017/S0953756299002191,
- Z.Kabir, I. P. O’Halloran, J.W. Fyles et C. Hamel, ‘ Seasonal changes of arbuscular mycorrhizal fungi as affected by tillage practices and fertilization : Hyphal density and mycorrhizal root colonization », Plant and Soil , vol. 192! Number = 2, , p. 285-293
- Borowicz, V.A., « Do arbuscular mycorrhizal fungi alter plant–pathogen relations? », Ecology , vol. 82, n O 11, , p. 3057-3068
- Jean Garbaye, Mycorrhizal symbiosis Editions are , p. 133-140 .
- (in) B. A. D. Hetrick, G. W. T. Wilson & T. S. Cox, ‘ Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors » , Canadian Journal of Botany , vol. 70, n O 10, , p. 2032–
2040 (DOI 10.1139/b92-253 ) . - Jean Garbaye, Mycorrhizal symbiosis. An association between plants and mushrooms Which, , p. 97
- Peter M., Ayer F. & Egli S. 2001. Nitrogen addition in a Norway spruce stand altered macromycete sporocarp production and below-ground ectomycorrhizal species composition. New Phytologist 149: 311-325
- (Newton et Pigott, 1991)
- Perry P.D. 1995. Self-organizing systems across scales. Trends in Ecology and Evolution 10: 241-244,
Kranabetter, J.M., & Wylie, T. 1998. Ectomycorrhizal community structure across forest openings on naturally regenerated western hemlock seedlings. Canadian Journal of Botany 76: 189-196.,
Jones M.D., Durall D.M. & Cairney W.G. 2003. Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. Tansley review. New Phytologist 157: 399-422.,
Lazaruk l., Kernaghan G., Macdonald S.E. & Khasa D.P. 2005. The impact of partial forest harvesting on the ectomycorrhizae of Picea glauca in northwestern Alberta. Canadian Journal of Forest Research, 35: 1-13 - Waters J.R., McKelvey K.S., Zabel C.J. & Oliver W.W. 1994. The effects of thinning and broadcast burning on sporocarp production of hypogeous fungi. Canadian Journal of Forest Research 24: 1516-1522, McKelvey K.S., Zabel C.J. & Oliver W.W. 1994. The effects of thinning and broadcast burning on sporocarp production of hypogeous fungi. Canadian Journal of Forest Research 24: 1516-1522
- Egli S. & Ayer F. 1997. Is it possible to improve the production of edible mushrooms in the forest? The example of the Cheneaz mycological reserve in Switzerland. French forest review 49: 235-243)
- Le Tacon F., Delmas J., Gleyze R. & Bouchard D. 1982b. Influence of the water diet of the soil and fertilization on the fruiting of the black truffle of Périgord (Tuber Melanosporum Vitt.) In the south-east of France. ACTA OECOLOGICA 3-4: 291-306.
Wiklund K., Nilsson L.O. & Jacobson S. 1995. Effect of irriguation, fertilization and artificial drought on basidioma production in a Norway spruce stand. Canadian Journal of Botany 73: 200-208. - (of) Frank A.B., 1885, “About the diet of certain trees based on root -symbiosis by underground mushrooms” (Sur La Nutrition de Certains arbres par l’Intermédiaire de la symbiosis entrre les racines et des mushroom souterrain),) Reports of the German Botanical Society , 3, p. 128–145
- (in) James M. Trappe ,, ‘ A.B. Frank and mycorrhizae: the challenge to evolutionary and ecologic theory » , Mycorrhiza , vol. 15, n O 4, , p. 277–281 (DOI 10.1007/s00572-004-0330-5 ) .
- (in) Marcel G. A. van der Heijden, Francis M. Martin, Marc -André Selosse, Ian R. Sanders, ‘ Mycorrhizal ecology and evolution: the past, the present, and the future » , New Phytologist , vol. 205, n O 4, , p. 1406-1423 (DOI 10.1111/NPH.13288 ) .
- ‘ Harley, J. L. 1948. Mycorrhiza and soil ecology », Biological Review , vol. 23, n O 2, , p. 127-158 (DOI 10.1111/j.1469-185X.1948.tb00460.x ) .
- (in) J.L. Harley, S.E. Smith, Mycorrhizal symbiosis , Academic Press, , 483 p. .
- Francis Martin, Under the forest. To survive you need allies , Hump , p. 121 .
- (in) Barbara moved, ‘ The establishment of vesicular-arbusacular mycorrhiza under aseptic conditions » , Journal of general microbiology , vol. 27, n O 3, , p. 509-520 .
- (in) J.M. Phillips, D.S. Hayman, ‘ Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection » , Transactions of the British Mycological Society , vol. 55, n O 1, , p. 158-161 (DOI 10.1016/S0007-1536(70)80110-3 ) .
- (in) M. Gardes, T.D. Bruns, ‘ ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts » , Molecular Ecology , vol. 2, n O 2, , p. 113–118 .
- (in) Sumei li, yinglong chen, xiangui lin, runjin liu, ‘ Mycorrhizology in the 21st Century » , Journal of Fungal Research , vol. 10, , p. 182-189
Bibliography [ modifier | Modifier and code ]
- Jean-André Fortin, Christian Planchette, Yves Piché, The mycorrhizae – The new green revolution Editions that Multimondes, Quebec, 2008, 148 p.
- Victoria Gomez Roldan, ” Role of strigolactones in mycorrhizal symbiosis with shrub. “, PHD, Paul-Sabatier University, Toulouse, France, 2009.
- Garbaye J (2013) An association between plants and mushrooms, Quae editions, published 10/28/2013, (ISBN 9782759219636 ) , 280 pages.
Related articles [ modifier | Modifier and code ]
external links [ modifier | Modifier and code ]
Recent Comments