Nitrogen protoxide – Wikipedia
| Nitrous oxide | |
 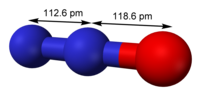 Nitrogen oxide structure. |
|
| Identification | |
|---|---|
| UICPA name | nitrous oxide |
| Synonyms |
nitrous oxide |
| N O CAS | |
| N O Echa | 100.030.017 |
| N O THIS | 233-032-0 |
| N O RTECS | QX1350000 |
| Code ATC | N01 |
| Pubchem | |
| Chebi | 17045 |
| N O AND | E942 |
| FEMA | 2779 |
| SMILES | |
| Inches |
|
| Appearance | Colorful liquefied compressed gas, characteristic odor [ 2 ] . |
| Chemicals | |
| Formula | N 2 O [Isomers] |
| Molar mass [ 4 ] | 44.012 8 ± 0,000 7 g/mol N 63.65%, O 36.35%, |
| Dipolar moment | 0.160 83 D [ 3 ] |
| Physical properties | |
| T° fusion | −90,8 °C [ 2 ] |
| T° boiling | −88.5 °C [ 2 ] Decomposition at 300 °C |
| Solubility | 1.5 g l −1 (water, 15 °C ) [ 2 ] . Also soluble in sulfuric acid, ethanol, ether, oils. |
| Volumic mass | 1.23 g cm −3 (liquid, −89 °C ) [ 2 ] 0.001 80 g cm −3 (Gas, 25 °C ) [ 5 ] |
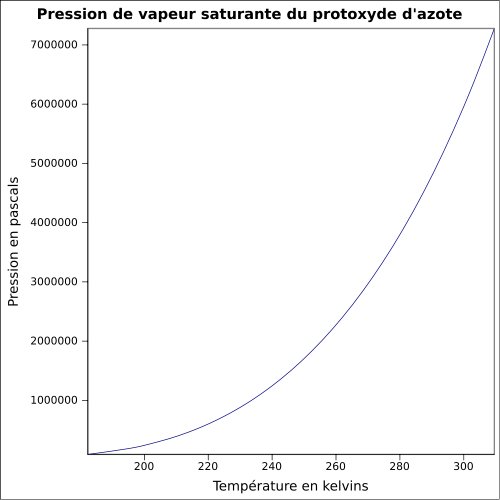
| Saturating steam pressure | 51.7 bar at 21 °C
|
| Critical point | 72.7 bar , 36.55 °C [ 7 ] |
| Speed of sound | 263 m s −1 ( 0 °C , first atm ) [ 8 ] |
| Thermochimie | |
| S 0 Gaz, 1 bar | 219.96 J mol −1 K −1 |
| D f H 0 gas | 82.05 kj times −1 |
| D vap H° | 16.53 kj times −1 ( first atm , −88,48 °C ) [ 9 ] |
| C p |
|
| PCS | 82.1 kj times −1 ( 25 °C , gas) [ ten ] |
| Electronic properties | |
| first re ionization energy | 12,886 this (gas) [ 11 ] |
| Precautions | |
| SGH [ twelfth ] | |
| H270 , H280 , H335 , P370 , P376 And P403
H270 : Can cause or worsen a fire; combur |
|
| Simdut [ 13 ] | |
   A, C, D2a, |
|
| NFPA 704 | |
|
|
|
| Transport [ twelfth ] | |
| Inhalation | Asphyxiating so pure inhaled |
| Skin | Toxic so cryogenic or compressed |
| Eyes | Toxic so cryogenic or compressed |
| Pharmacokinetic data | |
| ORANGE | 105 %vol [ Ref. desired] |
| Metabolism | No |
| Therapeutic considerations | |
| Therapeutic class | General, analgesic anesthetic |
| Administration | Inhaling |
| Psychotropic character | |
| Category | Dissociative hallucinogen |
| Way of consuming |
Inhaling |
| Other names |
Laughing gas |
| Risk of dependence | Pupil [Ref. necessary] |
|
|
|
| IS units and CNTP , unless otherwise stated. | |
| modifier |
|
The nitrous oxide , or Diazote monoxide , nitrous oxide , nitrogen hemioxide or laughing gas , is a chemical compound of formula n 2 O .
This colorless gas has a slightly sweet smell and taste. It is used in anesthesia, surgery, odontology, pediatrics as a adjuvant (in an equimolar mixture with oxygen) for its anesthetic and analgesic properties. It is said to be “hilarious gas” because euphorizing in inhalation, hence its use as a hallucinogenic recreational drug [ 14 ] , [ 15 ] . Like Comburant, it increases the power of motor competitions. With the acetylene H-C≡C-H , il est utilisé dans certains appareils d’analyse (spectrométrie d’absorption atomique[16]).
Its emissions are of natural and human origin (more than 20% increase in air since the pre-industrial era). Present in the state of traces in the dry air ( 330 games per billion [ 5 ] ), it is a powerful greenhouse gas ( 298 times more powerful than CO 2 ) et il est devenu le 1er contributeur à la destruction de la couche d’ozone[17]. Des analyses américaines publiées en 2021 (commandées par le président Joe Biden[réf. nécessaire]) concluent que son coût social a été sous-estimé, car ne tenant pas compte de l’appauvrissement de la couche d’ozone stratosphérique, effet qui à lui seul pourrait augmenter de 20 % sa valeur de nuisibilité sociale. Selon la même étude, ses liens avec d’autres effets de la pollution azotée pourraient rendre son « atténuation encore plus impérieuse » dans l’atmosphère[18].
Nitrogen oxide is prepared by decomposition of the melted ammonium nitrate between 250 °C And 260 °C According to the following reaction equation [ 19 ] :
- NH 4 NO 3 → N2O + 2 H2O
There is always 1 to 2% of nitrogen n 2 and nitrogen monoxide no. The latter is eliminated by passing in iron sulfate (II). The ammonium nitrate used must be free from chloride ions – which catalyze the formation of n 2 . However, heating ammonium nitrate solutions in nitric acid or sulfuric acid leads to pure nitrogen oxide, even in the presence of small amounts of chloride ions.
Very stable in the atmosphere, very little soluble in water, it is highly soluble in oils and in fatty substances.
Reactivity [ modifier | Modifier and code ]
This gas does not react to room temperature with the Dihalogens (cl 2 , Br2 ou I2), ni avec les métaux alcalins (Li, Na, K).
It can nevertheless be fixed on certain metal cations as a ligand and forms complexes like [RU (NH 3 ) 5 (N 2 O)] 2+.
It can also oxidize transition metals with low valence in complexes.
It is not modified by ozone o 3 , but contributes to the degradation of stratospheric ozone (layer of protective ozone of the biosphere against solar UV).
At high temperature, it breaks down into nitrogen and oxygen:
- 2 N 2 O → 2 N2 + O2
He reacts with many organic compounds and he boosts combustions thanks to his strong oxidizing power [ 2 ] .
He thus served as a comburant to boost the engines of high altitude fighter aircraft during the Second World War and those of competition cars (car tuning and dragsters). It can also cause violent accidental explosions and large damage in the overalls of municipal waste incinerator when high capacity cartridges of euphorizing gas are unfortunately leading to it.


In 1772, Joseph Priestley discovered nitrogen oxide [ 20 ] and describes it in Experiments and Observations on Different Kinds of Air .
In 1798, Humphry Davy discovered, among other things, its euphoric properties. Nitrogen oxide is used at the end of XVIII It is century as “hilarious gas” in fairs.
In 1844, the dentist Horace Wells discovered his anesthetic effects, which he experienced on himself. Unfortunately, impatient to publicize his discovery, Wells launches without prior in -depth experimentation, in demonstrations in front of a medical audience in Hartford (Connecticut) and Harvard, who are resounding failures. Disappointed, Wells definitively abandons dentistry. We will have to wait for the successful research and demonstrations of William Thomas Green Morton with ether (October and November 1846 in Boston) so that anesthesia was used by surgeons, which allowed surgery to make a leap forward (pain was one of the two large limits of surgery, with the infection). Nitrogen oxide is restored by a so -called Colton, a show of hilarious gas shows, who, associated with dentist J.H. Smith, install a clinic in New York and then export their process in Europe.
In 1961, English doctors used it for the first time in obstetrics where he attenuates pain and had an anxiolysis role.
In the 1980s, its use was greatly improved by the association with oxygen because the first risk in its use remains asphyxiation for lack of oxygen. Medicine therefore uses it as a team mixture with oxygen (meopa). Nitrogen oxide gradually replaces ether and chloroform in obstetrics [ 21 ] .
In 1998, he received an analgesic drug status.
In , he obtained in France the marketing authorization (AMM), while it is already regularly used in this form during the XX It is century. Previously, it was distributed (outside the classification medication like other gas medical ) in the majority of operating blocks and was one of the general anesthesia agents during the XX It is century ; Its low anesthetic power restricting its use to not very painful acts, and the role of adjuvant of more powerful drugs which it makes it possible to reduce doses by reducing certain undesirable effects.
At the end of 2009, in France, Afssaps changed the regulations so that the Meopa can be used outside hospitals [ 22 ] , Regularizing the situation of those who used it (in France at least since 1996 “at home” in dull adult patients to relieve the pain of skin ulcers associated with Kaposi syndrome and in certain elderly patients carrying skin ulcers or Sunckets made too sleepy by previous treatment, or sporadically in home pediatrics, to relieve major pain when morphine products were no longer enough in children in palliative care [ 22 ] . It was also used punctually by doctors, for example in dermatology or ENT at the home of adult patients, then very regularly in children ( Annex 2 ) without reimbursement by social security because this analgesic means was outside the nomenclature).
Doctors report that “The reproducibility of the effect is not complete, with a lack of efficiency in some” ; The main side effects are nausea and vomiting, “Generally reversible in a few minutes when you stop treatment” [ 23 ] (a group of experts [ 24 ] gathered by Afssaps considers in its report [ 25 ] what “The Meopa does not cover all the pain and painful care. According to the indications, the age of the child and the experience of the team, 10 to 30% of failures are observed. Children under two have much less marked effects ” [ 23 ] ).
Afssaps specifies that
“Any misuse or abuse must be warned. In this context, a reclassification in the category of drugs reserved for professional use has been retained; This gas mixture can therefore only be distributed to the health professionals concerned and not directly to patients [ 21 ] .
The modification of the conditions of prescription and issuance led to the complete revision of the RCPs of the specialties concerned. In addition, given all the risks linked to the use of specialties based on Meopa, Afssaps conditions their provision outside health establishments to the implementation of a management plan for Common national risks (PGR).
It is based on a laboratory commitment to the implementation of management and minimization measures of the following risks [ 21 ] :
- the realization of a reinforced pharmacovigilance and pharmacodependence monitoring [ 26 ] with
- the incitement of health professionals to notify the undesirable effects, the cases of abuse, pharmaco dependence, diverted use and misuse linked to the use of Meopa;
- During the first two years, the semi -annual transmission to the Afssaps of up -to -date periodic reports of pharmacovigilance accompanied by the French summary of reported cases, the consumption assessment and the conditions of use of the product;
- securing and traceability of distribution and recovery, with in particular verification in ordering the quality and training of the applicant;
- Securing and traceability of use: volumes of bottles limited to five liters and securing bottles;
- The realization of a training plan for professionals: doctors, pharmacists and nursing staff;
- The provision of an information document for patients (in case of home storage.). »»
The following year (2010), the hospital reserve opens up to dentistry. Afssaps is launching national monitoring of pharmacovigilance and pharmacodependence of the N 2 O ; en particulier, les vols de N2O doivent lui être déclarés[21].
In 2001 (in France), the indications in hospitalization at the pediatric home joined the following three cases:
- invasive and repeated medical acts, insufficiently calmed by other weak analgesic means (EMLA, analgesic landing 1 ) such as intramuscular injections for chemotherapy, repairing dressing on injured skin [ 21 ] ;
- Medical care deemed normally not very painful by doctors, but source of anxiety or anxiety marked for a particular child (such as central venous catheter dressings, blood peripheral venous blood samples) [ 21 ] ;
- repeated care for months becoming a source of marked difficulties in acceptance [ 21 ] .
According to OMM (2017), its emissions are of natural origin for around 60% and human for around 40% [ 27 ] While an earlier estimate (2009) concluded 30% of human origin [ 28 ] .
Natural sources [ modifier | Modifier and code ]
Some soil and ocean microorganisms are the main natural sources, but it is also produced by the combustion of organic matter and fossil fuels, industry or wastewater treatment stations [ 29 ] , etc. Its production in soils and in air from soils is strongly increased by nitrogen fertilization (use of fertilizers, including natural “organic” origin, and nitrogen amendments) [ 30 ] . Part of the emissions from cultivated or meadow soils that have been the subject of spreading manure and slurry, purification sludge or certain fertilizers is thus of human origin [ thirty first ] .
Bacteria living in some anthill are an important source; The gases exhaled by twenty-two nests of fumes cutting leaves (from the southwest of Costa Rica) showed that in wet and poor oxygen context, these bacteria produce very large quantities of methane and nitrous oxide [ 32 ] . These quantities are comparable to those observed in wastewater treatment plants and slurry pits [ 32 ] . These ants nevertheless also contribute to the functions of carbon wells of the soil; The results of their global climate effects are not yet evaluated, due to lack of sufficient data [ 32 ] , but this production explains why previous studies had measured very variable levels of methane and nitrous oxide in the forests and regions where these ants build their underground nests [ 32 ] .
Human sources [ modifier | Modifier and code ]
A third of the n 2 O de l’atmosphère provient de l’épandage de lisier et d’engrais azotés[33],[34]. Une autre contribution importante est la synthèse d’acide adipique, HO2C(CH2)4CO2H, un précurseur de nombreux polymères de nylon et qui dégage un N2O pour chaque molécule d’acide adipique[35],[36].
En France, l’agriculture contribue à hauteur de 86 % aux émissions de N2O provenant essentiellement de la transformation des produits azotés (engrais, fumier, lisier, résidus de récolte) épandus sur les terres agricoles. Une petite partie des émissions provient de la pollution routière, en particulier des véhicules équipés de pots catalytiques et de quelques procédés industriels[37],[38].

Les sources de moindre importance (ex. : eaux usées et aquaculture) figurent dans la barre du bas intitulée « Autres ». Les barres rouges correspondent aux marges d’erreur (rouge)[39]. En 2005, le total (dominé par les émissions agricoles) correspond à 30-40 % environ de tout le N2O introduit annuellement par l’humain dans l’atmosphère.
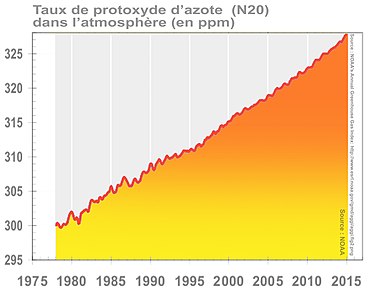





Nitrogen oxide is also a powerful greenhouse gases [ 42 ] . Its content increases in the air at least from the middle of XVIII It is century with an acceleration at the start of XX It is century and high acceleration from 1970s [ 43 ] .
According to the IPCC [ 44 ] , its potential for global warming at a hundred years is equal to 298, that is to say that it contributes 298 times more to global warming than the same mass of CO 2 émise en même temps pendant les cent ans qui suivent leur émission.
In 2009, in an article in the journal Science entitled “Nitrogen protoxide: nothing to laugh” [ 45 ] , Donald J. Wuebbles (department Atmospheric Sciences, School of Earth, Society, and Environment of the University of Illinois) is surprised that despite its importance long recognized as greenhouse gas and destructive of the ozone layer, nitrous oxide sometimes still seems to remain forgotten gas in Questions of protection of the atmosphere, climate or ozone layer. We mainly continue to focus on carbon dioxide emissions (Co 2 ) et de méthane (CH4) provenant notamment des combustibles fossiles en oubliant que « les changements futurs du climat et de la répartition de l’ozone stratosphérique dépendent des émissions et de la concentration changeante de N2O dans l’atmosphère […] l’oxyde nitreux mérite beaucoup plus d’attention et de considération pour une action politique visant à contrôler les futures émissions anthropiques »[45].
Its concentration in the earth’s atmosphere has reached 329 ppb In 2016, or +22% compared to the pre-industrial level, contributing 6% to radiative forcing induced by persistent greenhouse gases (see graph opposite) [ 27 ] .
Contribution to the destruction of the ozone layer [ modifier | Modifier and code ]
Nitrogen oxide (n 2 O ) en excès contribue à la destruction de la couche d’ozone, en interaction avec d’autres gaz[46]. Il est considéré comme ayant une longue durée de vie dans la haute atmosphère (comme le CO2 et le méthane responsable en excès, à l’effet de serre[47]. Or, le réchauffement peut exacerber le trou de la couche d’ozone et inversement[47]).
From the early 1970s, meteorologists and scientists were concerned about the increase in the N 2 O dans la haute atmosphère[48], mais il n’a pas été inclus dans le Protocole de Montréal. Au début du XXIe siècle, à la suite du recul des émissions des gaz soumis au Protocole de Montréal, le N2O devient le premier ennemi de la couche d’ozone[17],[28],[39]. Le GIEC prévoit qu’il devrait continuer à s’accumuler dans l’atmosphère[47], tendance confirmée par un article publié près de dix ans plus tard, en 2018 dans Science, et il devrait le rester durant tout le XXIe siècle[28] car la part de protéines animales dans l’alimentation humaine ne cesse de croître, associée à une production de fumier et de soja également source croissante d’oxyde nitreux. Selon un article de la revue Nature Geoscience, le lisier et le fumier libèrent ainsi dans l’air 2 % environ de l’azote qu’ils contenaient et les engrais azotés 2,5 % ; ces deux sources introduisent dans l’atmosphère 2,8 Mt de N2O pour les lisiers et 2,2 Mt pour les engrais synthétiques (dont la production et le transport et l’épandage produisent aussi par ailleurs du CO2 qui aggrave l’effet de serre. L’industrie rejette bien moins de N2O (0,8 Mt/an environ) et la combustion de la biomasse (0,5 Mt/an).
De 1860 à 2005, le taux de N2O dans l’air serait passé de 270 à 319 ppb (parties par milliard, un taux de croissance assez comparable à celui du CO2).
Various strategies for attenuation of n 2 O sont possibles, dont en agriculture, où des changements techniques et de comportements pourraient considérablement réduire les émissions de N2O (et d’autres formes d’azote réactif), mais elles sont peu soutenues par l’industrie et les États[39]. Les parties signataires du régime de l’ozone qui doivent appliquer la Convention de Vienne (1985) au travers de son Protocole de Montréal de 1987 pourraient aussi prendre des mesures pour gérer le N2O à l’avenir[39]. En 2017, par un amendement au protocole, elles ont intégré les HFC mais n’évoquent toujours pas le N2O. Des voies réglementaires claires permettraient pourtant de l’inclure dans le régime de protection de la couche d’ozone en partageant l’autorité sur le N2O avec les traités internationaux (actuels et futurs) sur le climat. Selon Kanter et al. (2012), ce serait un moyen supplémentaire et précieux dans la gouvernance et diplomatie du développement durable[39].
In some contexts (certain industrial premises, operating room in hospitals, use ” recreational In poorly ventilated premises or habitacles), it is an indoor air pollutant [ 49 ] .
It is used in the context of local anesthesia, generally during operations outside hospitals (emergency cases) or in the event of contraindications.
This gas is also popular with certain evenings where its effects are diverted to provoke sensations of uncontrolled giggles in the inhaleur.
Medical use [ modifier | Modifier and code ]
Nitrogen oxide has an analgesic effect (soothes pain) and potentia (increases) the effect of anesthetic drugs administered at the same time. We therefore use it:
- in anesthesia, as a common component of combined general anesthesia, in combination with injectable (hypnotic, morphine, curares) or inhaled anesthetics;
- in emergency medicine (fracture or dislocation reductions), pediatrics or in the delivery room, in the form of an equimolecular mixture with dioxygen (meopa);
- For the practice of painful gestures (meopa), in particular in children: difficult blood sample, myelogram, lumbar puncture, etc. Here it has the advantage of often inducing an amnesia of the gesture, useful when it should be repeated. For increased efficiency, nitrogen oxide is here associated with the preventive intake of analgesics, of the morphine most often.
Nitrogen oxide (MEOPA) is classified in the model of essential drugs of the World Health Organization (update list in ) [ 50 ] , but he does in a hospital environment “The object of reinforced monitoring of pharmacovigilance and addictovigilance” [ 26 ] .
Action mechanism [ modifier | Modifier and code ]
The pharmacological mechanism of action of the N 2 O en médecine n’est pas encore complètement compris.
The N 2 O interfère avec des voies de signalisation en modulant directement une large gamme de canaux ioniques commandés par des ligands (récepteurs ionotropes), ce qui jouerait un rôle majeur dans bon nombre de ses effets. Il bloque modérément les canaux du récepteur nicotinique de l’acétylcholine contenant les sous-unités NMDA et la sous-unité β2 (dite « CHRNB2 » pour neuronal acetylcholine receptor subunit beta-2). Il inhibe faiblement les récepteurs AMPA, et kaïnate, GABAA-rho (anciennement nommé « récepteur GABAC ») et le récepteur 5-HT3. Et il potentialise légèrement les récepteurs GABAA et glycine[51],[52].
Il active le domaine K+ (canal potassique) à deux pores[53].
Bien que le N2O affecte (très rapidement) de nombreux canaux ioniques, ses effets anesthésiques, hallucinogènes et euphorisants sont probablement principalement ou totalement induits par une inhibition des courants médiés par les récepteurs NMDA[51],[54].
In addition to its effects on ion channels, the N 2 O pourrait agir en « mimant » l’oxyde nitrique (NO) dans le système nerveux central ; ceci pourrait expliquer une partie au moins de son caractère analgésique et anxiolytique[54] (remarque : l’oxyde nitreux est trente à quarante fois plus soluble que l’azote).
Les effets de l’inhalation de doses sous-anesthésiques d’oxyde nitreux varient selon plusieurs facteurs, et avec des différences individuelles[55],[56], cependant selon Jay (2008), il est reconnu pour induire les états et sensations suivants[57] :
After inhalation of n 2 O , une minorité de personnes présenteront également des vocalisations incontrôlées et des spasmes musculaires. Ces effets disparaissent généralement quelques minutes après l’élimination de la source d’oxyde nitreux[57].
Euphoric effect [ modifier | Modifier and code ]
In the laboratory rat, the n 2 O stimule la voie méso-limbique de récompense, en induisant la libération de dopamine et en activant dans le cerveau les neurones dopaminergiques de l’aire tegmentale ventrale et du noyau accumbens, vraisemblablement par antagonisation des récepteurs NMDA localisés dans le système[58],[59],[60],[61]. Cette action expliquerait les effets euphorisants du N2O et semble aussi notamment augmenter ses propriétés analgésiques[58],[59],[60],[61].
It is remarkable, however, that in laboratory mice, the n 2 O bloque la libération de dopamine induite par les transporteurs liés aux amphétamines dans le noyau accumbens et qu’il bloque l’addiction, supprime le conditionnement pavlovien de type PPC (Préférence de place conditionnée/Conditioned place preference) induit par la cocaïne ou la morphine, sans produire d’effet de renforcement ni d’aversion[62],[63].
The effects of N 2 O sur le PPC chez les rats sont plus complexes et « mélangés », consistant en un renforcement, une aversion et aucun changement[64].
On the other hand, the N 2 O est un facteur de renforcement positif chez le singe-écureuil[65]. Et il est bien connu pour être addictif chez l’humain[66].
These differences in response according to the species at n 2 O sont mal comprises : elles pourraient refléter de réelles variation entre espèces proches, voire au sein d’une espèce (ou refléter des différences méthodologiques entre études ?)[63].
Des études cliniques humaines ont conclu que N2O induit chez l’humain des réponses mixtes, similaires à celles décrites chez les rats, reflétant une variabilité subjective qui semble élevée[67],[68].
Anxiolytic effect [ modifier | Modifier and code ]
Anxiety behavioral tests show that a low dose of n 2 O est efficacement anxiolytique. Cet effet « anti-anxiété » a été associé à une activité accrue des récepteurs GABAA, car il est partiellement inversé par les antagonistes de ces récepteurs.
Conversely, animals that have developed tolerance for anxiolytic effects of benzodiazepines are then also partially tolerant at N 2 O [69]
Clinical studies have shown that a human receiving 30% of N 2 O dans l’air inhalé, les antagonistes des récepteurs aux benzodiazépines (GABAA) réduisent le nombre de rapports subjectifs de sensation de « sensations fortes », sans toutefois altérer les performances psychomotrices[70].
Analgesic effects [ modifier | Modifier and code ]
These effects seem to be linked to the interaction between the “endogenous opioid system” and the “descending noradrenergic system”. In effect :
- When animals receive morphine chronically, they develop tolerance for its effects Pain killer , and they are also also tolerant to the analgesic effects of the N 2 O [71] ;
- The administration of antibodies which bind and block the activity of certain endogenous opioids (but not β-endorphin) also blocks the antinocceptive (painkling) effects of the N 2 O [72] ;
- Drugs that inhibit the degradation of endogenous opioids also potentiate the antinocceptive effects of the N 2 O [72] ;
- Several experiments have shown that antagonistic drugs of opioid receptors applied directly to the brain block the antinocceptive effects of the N 2 O , mais que ces mêmes médicaments n’ont aucun effet s’ils sont injectés dans la moelle épinière. À l’inverse, les antagonistes des récepteurs α2-adrénergiques (adrénorécepteurs) suppriment les effets de réduction de la douleur induits par le N2O s’ils sont administrés directement dans la moelle épinière, mais pas lorsqu’ils sont appliqués directement au cerveau[73].
- Mouses or “Knock-out” animals for α2B-Adrenocers (or “ALPHA-2B adrenergic receiver”) not producing noradrenaline are made almost completely resistant to antinocceptive effects of N 2 O [74].
Apparently, the release of endogenous opioids induced by the N 2 O provoque la désinhibition des neurones noradrénergiques du tronc cérébral, laquelle libère de la noradrénaline dans la moelle épinière et inhibe le signal de douleur[75]. En 2019, la manière dont le N2O provoque la libération de peptides opioïdes endogènes n’est pas encore comprise. Les progrès de la neurophysiologie et de la cartographie du cerveau et de ses fonctions sensorimotrices, cognitives et langagières pourraient à l’avenir permettre de mieux comprendre ces phénomènes (pour les effets « hilarants » du N2O y compris).
Combur in internal combustion engines [ modifier | Modifier and code ]
Principle [ modifier | Modifier and code ]
Due to its higher oxygen content than air, nitrogen oxide is sometimes used as an extra or substitution for the latter in internal combustion engines [ 76 ] . It increases the combustion/fuel load in the cylinder, to promote combustion, and thus to significantly increase the power of the engine (from 30% to approximately 100%). It is the nitrogen oxide engine.
Aviation [ modifier | Modifier and code ]
The injection of nitrogen is used during the Second World War in certain German combat aircraft. A device, called “GM-1”, aimed to compensate for the decrease in air dioxygen at altitude (gas taken from air, used as comburant by piston motors), which had the consequence of reducing the level to combine in the engine compared to fuel, and thus led to the drop in the power delivered by the engine as well as the increase in consumption [ 77 ] . The injection of nitrogen oxide therefore aimed to compensate for the lack of comburant in the engine so as to allow it to operate at high altitude with an efficiency identical to that of low or medium altitude. The pilot thus had a power reserve which he could use until the candy containing the nitrogen oxide in liquid form, about ten minutes.
At the time, these systems were poorly controlled and required a great precaution of use, especially for complicated engines of German devices. A pilot wishing to use the GM-1 had to do so at an altitude where the air was already rarefied (from around 6,000 m altitude) and had to reduce the gases before restarting them once the device started, under penalty of breaking the engine or worse, to explode the aircraft.
Automobile competition [ modifier | Modifier and code ]
Later, and like the other overeating processes such as the compressor and the turbo, the principle of the injection of nitrogen oxide was taken up in automotive competition, then by the individual since we find on the market of the kits nos ( nitrous oxide systems ) that can be adapted to just any automobile. Although these kits are very popular with car tuning enthusiasts, their installation on standard vehicles remains illegal in many countries. Use in France remains authorized as long as the (compulsory) safety valve valve is locked and that the equipped vehicle is not used on a public road; However, insurance is entitled to refuse to take charge of such vehicles. The presence of a nitro kit during an accident causes a significant increase in risks. In the event of shock, temperature change, wear, use of a poor quality kit, or non -renewal of connections and change of bottles in accordance with the standards governing the general gas conditions, that the Installation of the kit is not made a professional accustomed to these systems, there is a risk of gas leaks or explosion of the containers and their consequences (leak: risk of increased fire, spontaneous fire (self-irritation, Dangerous chemical reaction), cold burns, asphyxiation, loss of knowledge, degradation/degradation of the mechanical parts of the car.
In the event that there is a rupture of nitro bottles, is added to the risk linked to gas leaks, the explosion risks of the vehicle, the explosion/explosion being multiplied compared to a deflagration without nitro kit. Fuel (petrol, diesel, etc. ) Burns and detonates faster, the temperature of the flames is about twice higher.
Use as a so -called “recreational” drug [ modifier | Modifier and code ]
Nitrogen oxide, legally sold in local shops – theoretically for whipped cream siphons – conditioned in candy or oval steel cartridges, has been, since 2000 at least, diverted for recreational purposes for its psychodysleptic properties [ 78 ] .
According to Garbaz, the nitrogen oxide drug addiction has stayed for a long time (although from the XVIII It is century, he had parties often organized to the homes and by wealthy bourgeois (obtaining gas was done by chemical reaction directly on the spot) whose main goal was the inhalation of nitrogen oxide) rare, not touching First that employees of the hospital or medical world, then an increasing number of young adults and adolescents, even children. By extending to the general public (via the great availability of low -cost cartridges), “It exposes to the occurrence of accidents and undesirable unknown effects” [ 79 ] . This diversion of nitrogen oxide has been proven in the United States and the United Kingdom since the start of 1980s and in France since 1996/1998 (first cases reported by the Marseille anti -paint center), in student circles in particular [ 20 ] . It is often shared or sold on the occasion of techno evenings, or in free parties, technivals, trance evenings, etc. [ 80 ] .
If it is taken alone and episodically, nitrogen oxide does not represent a risk of addiction: the n 2 O fait rire (même si en réalité, bien que surnommé « gaz hilarant », il induit moins souvent le rire que les boissons alcoolisées ou le cannabis), son effet est de relativement courte durée et certaines personnes ne ressentent peu ou pas d’effets anesthésiants, ni d’effet agréable, et il n’induit généralement pas de dépendance. Ce n’est cependant pas un produit anodin. Il peut induire des lésions irréversibles ou des pathologies graves telles qu’une forme d’anémie particulière, d’atteintes neurologiques (poly-neuropathies, d’ataxies, c’est-à-dire troubles de l’équilibre, problèmes de coordination motrice), note William Lowenstein, spécialiste en médecine interne et addictologie, président de SOS Addictions[80]. Et selon les statistiques anglaises collectées par un rapport de l’université de Londres (cité par le quotidien The Independent), en six ans, de 2006 à 2012, dix-sept jeunes Britanniques sont morts après avoir consommé du gaz hilarant, dont six par asphyxie (hypoxie)[80].
Nitrogen oxide seems to be subject to modes ; In France, a first peak in consumption appeared towards the an 2000 [ 80 ] and a revival noted in 2017-2018, especially in the regions near the United Kingdom [ 81 ] . It is most often inhaled by mouth via inflatable rubber balloons or condoms inflated with gas (sold by unit), according to a French report of 2007 commanded by the Directorate of Health [ 82 ] . Balloon packaging makes it possible to avoid frostbite and pulmonary or brain embolisms caused by the cold due to the relaxation of the gas if it is “Directly sucked in the cartridge” , like a bomb of dusting gas or a siphon in Chantilly, note drugs info service, which adds that “The fleeting effects of nitrogen oxide sometimes encourage user to repeated inhalations that can lead to death by asphyxiation” [ 80 ] .
“Unlike other drugs, there is no dependence on nitrogen oxide” , indicates info service drugs. [Ref. necessary]
Dependence cases are exceptional. People with real dependence (in the pharmacological sense) in nitrogen oxide, often present an initially a disease that is not relieved by the usual treatments and in these situations the N 2 O Present more benefits than risks (terminal cancer in children, chronic lesions and ulcers in elderly patients or in people victims of the HIV or, during chronic pain syndromes).
In rare cases where dependence begins as a result of so -called “recreational” consumption, consumers quickly find themselves in a situation of social isolation which is often accompanied by economic difficulties, sources of malnutrition (deficiencies).
“Even if it can be scary, you have to put into perspective. Hilarious gas is only a small fashion, in young people, in a search for drunkenness which is timeless ” , selon William Lowenstein. [Ref. necessary]
In France [ modifier | Modifier and code ]
In 2011, the National Agency for Drug Safety and Health Products (ANSM) warned against the recreational use of volatile substances in general and nitrogen oxide in particular (5.5% of them said having already experienced it in 2011) [ 80 ] .
In 2014, Drugs Info Service had received very few requests for help or information (fifteen requests on 44,000 calls) [ 80 ] .
Following a strong resurgence in 2017, 2018 and 2019 in the North [ 83 ] , [ 84 ] , then in Île-de-France [ 85 ] and to the rest of the territory [ eighty six ] , [ eighty seven ] , a bill [ 88 ] tending to protect minors from the dangerous uses of nitrogen oxide is adopted unanimously on December 11, 2019 in the Senate [ 89 ] . The flagship measure is the prohibition of sales to minors, including on online commerce sites. The text also proposes to penalize the incitement of a minor to make a diverted use of a current consumption product to obtain psychoactive effects. He also plans to support the prevention policy carried out at school. The text should be submitted to the National Assembly in 2020.
Through a decree, the commune of Toulouse prohibited, the , the recreational use of hilarious gases on the public domain and the sale to minors [ 90 ] .
Nuisances and damage caused to municipal incinerators [ modifier | Modifier and code ]

N cartridges 2 O abandonnées dans la nature et dans les rues constituent une source de nuisances et de pollution non négligeable. En Belgique, rien qu’en trois semaines au mois de janvier 2023, Bruxelles propreté en a récolté plus de deux tonnes le long des voiries régionales auxquelles il convient également d’ajouter les collectes communales et celles des déchets ménagers habituels[91]. On les trouvent surtout aux abords des bars et des dancings.
Large capacity cartridges not completely emptied, if collected and cremated, give rise to violent explosions seriously damaging the ovens of household waste incinerators [ 92 ] . The bars of the ovens of ovens are breaking and the ashes agglomerate in the reception space located below the gates, which clogs the evacuation systems of the ashes (floor ash (in) ) [ 93 ] , [ ninety four ] . This is how many incinerators must frequently stop some of their ovens in order to be able to repair the often considerable damage. Agencies responsible for public cleanliness and the treatment of household waste are forced to carefully sort the garbage cans located on the public highway in order to protect the ovens of incinerators and to limit their unavailability linked to the time necessary for the repair of the damage caused by these explosions . The problem also affects many municipalities in France [ 93 ] , [ ninety four ] .
Other uses [ modifier | Modifier and code ]
Nitrogen oxide is used as propellant gas, especially in whipped cream candles.
It is also used in dry air bombs for electronics and computers. Its European code is the E942 [ 95 ] .
Dissolved in water, nitrogen oxide has a sweet taste [ 96 ] .
It was used for the conservation of meat [Ref. necessary] .
The effects of chronic exposure to low doses are poorly understood, but have been studied for certain exposed trades (anesthesists in surgery, for example [ 97 ] ).
In high doses, it causes neurological effects (spasticity with spasticity) and macrocytic anemia, with reduction in the rate of vitamine B twelfth circulating. Smith, in a review of health risks in hospital staff, recalls that it is a mitotic poison, which has been made responsible for tumors of the lymphoid system and the reticulo-endothelial system in the staff working in the Operation (with exchanges between chromatids sisters according to Saras et al. 1992, Eroglu et al. ) in which we have described various cytogenetic damage (problems also posed by exposure to other anesthetics).
Nitrogen oxide with high concentration can kill by asphyxiation (due to a lack of oxygen); The anesthetic mixture still contains at least 21% oxygen (the proportion of oxygen of the ambient air, and up to 50% in the case of meopa).
The diverted and prolonged use of nitrogen oxide presents, in addition to the risks of asphyxiation, the risks of medical complications for the newborn in the event of use during a pregnancy [ 98 ] .
Effects and consequences, toxicity [ modifier | Modifier and code ]
Short -term effects are fast and fleeting. They start fifteen to thirty seconds after absorption and end after two to three minutes.
In high doses, nitrogen oxide becomes narcotic with it, as possible effects [ Ref. desired] :
- Euphoria, feeling of well-being and laughter;
- Disinhibition;
- floating effect;
- visual and auditory distortions;
- feeling of increased members;
- modification of the voice, which becomes very serious (inverse effect of helium);
- memory losses;
- spasms;
- HyperSalivation [Ref. necessary] .
Significant short -term dose effects [ modifier | Modifier and code ]
Long -term large or repeated dose effects [ modifier | Modifier and code ]
The long -term toxic or undesirable effects of repeated exposure to nitrogen oxide are of several orders: hematological, neurological (cf. demyelination), psychiatric and teratogenic [ 79 ] .
Professionals are not the only ones concerned. Intense and/or regular recreational use of nitrogen oxide can cause symptoms and diseases that doctors are not accustomed to suspect or diagnose:
- Vitamin B deficiency twelfth (cobalamine) [ 100 ] Because the n 2 O oxyde les ions cobalt de cet oligoélément essentiel[101],[102] ;
- a reduction in vitamin B levels twelfth current [ 103 ] by inhibition of methionine hepatic synthase [ 104 ] , leading to large consumptions of n 2 O des neuropathies (troubles neurologiques) telles que des tremblements ou des difficultés à coordonner ses mouvements ;
- neurological complications are possible and unknown (even doctors [ 102 ] ), including the bone marrow and the spinal cord resulting in a symmetrical and progressive weakness of the lower limbs, equilibrium disorders and an increasing difficulty in walking, underlined by several recent studies [ 105 ] , [ 106 ] , [ 107 ] , [ 108 ] , [ 109 ] in people used to inhaling high doses of n 2 O . C’est le déficit en vitamine B12 qui entraîne la neurotoxicité du N2O à forte dose ou en cas d’usage récréatif chronique[110] : une démyélinisation des fibres nerveuses, ce qui finit par interrompre la transmission nerveuse (en l’absence de B12, le recyclage de l’homocystéine en méthionine est interrompu, empêchant la méthylation des protéines de la gaine de myéline, ce qui entraine une démyélinisation)[1]. Une sclérose de la moelle se combine à une atteinte dégénérative de la moelle épinière (visibles sur l’imagerie par résonance magnétique (IRM), où une coupe sagittale montre comme conséquence d’une dégénérescence subaiguë de la moelle un hypersignal en séquence pondérée T2 localisé préférentiellement au niveau des cordons postérieurs de la moelle cervicale ou dorsale[1]). Cette démyélinisation dégrade la sensibilité profonde[1]. En , à Londres des urgentistes et neurologues de divers hôpitaux ont rapporté dix cas de « sclérose combinée de la moelle » (SCM) chez des jeunes de 17 à 26 ans dont la plupart ne consommaient pas d’autres drogues (et pas d’alcool)[1],[111]. La concentration sanguine en vitamine B12 peut être normale, mais la vitamine non fonctionnelle. Une augmentation anormale du taux d’acide méthylmalonique (AMM) et d’homocystéine peuvent alors orienter le diagnostic[1] ;
- Quick vitamin B supplementation twelfth Conversely degeneration, but neurological recovery can be incomplete, especially if the person continues to inhale from N 2 O [112] ;
- A pneumothorax. Cases linked to nitrogen oxide were first described as an anesthesia or laparoscopy accidents (Diffusion of the N 2 O dans la cavité péritonéale, puis vers la cavité pleurale)[79]. L’inhalation de N2O peut révéler un pneumothorax asymptomatique et l’aggraver (« Les conséquences peuvent être dramatiques lors de son utilisation frauduleuse en milieu extra-hospitalier par méconnaissance de ses effets secondaires ») ;
- An anemia, said megaloblastic , because associated with abnormally large red blood cells [ first ] ;
- Psychological dependence.
- Marc Gozlan, “Nitrogen oxide, a hilarious gas that does not make doctors laugh at all” , Biomedical realities , December 28, 2018, The world (Accessed July 8, 2019).
- Nitrous oxide , International chemical security sheets
- (in) David R. Suffer , CRC Handbook of Chemistry and Physics , Boca Raton, CRC Press/Taylor & Francis, , 89 It is ed. , 2736 p. (ISBN 9781420066791 , Online presentation ) , p. 9-50 .
- Molar mass calculated after ‘ Atomic weights of the elements 2007 » , on www.chem.qmul.ac.uk .
- Information sheet , Gas Encyclopedia , Liquid air.
- (in) Robert H. Perry et Donald W. Green , Perry’s Chemical Engineers’ Handbook , USA, McGraw-Hill, , 7 It is ed. , 2400 p. (ISBN 0-07-049841-5 ) , p. 2-50 .
- (in) ‘ Properties of Various Gases » , on flexwareinc.com (consulted the ) .
- (in) William M. Haynes , CRC Handbook of Chemistry and Physics , Boca Raton, CRC Press/Taylor & Francis, , 91 It is ed. , 2610 p. (ISBN 9781439820773 , Online presentation ) , p. 14-40 .
- (in) David R. Suffer , CRC Handbook of Chemistry and Physics , Boca Raton, CRC Press/Taylor & Francis, , 90 It is ed. , 2804 p. (ISBN 9781420090840 , Online presentation ) .
- (in) David R. Suffer , CRC Handbook of Chemistry and Physics , Boca Raton, CRC Press, , 83 It is ed. , 2664 p. (ISBN 0849304830 , Online presentation ) , p. 5-89 .
- (in) David R. Suffer , CRC Handbook of Chemistry and Physics , Boca Raton, CRC Press/Taylor & Francis, , 89 It is ed. , 2736 p. (ISBN 9781420066791 , Online presentation ) , p. 10-205 .
- “Nitrous oxide” input in the chemical product database Achievement IFA (German organization responsible for occupational safety and health) ( German , English ) (Javascript required) .
- ‘ Nitrous oxide »In the chemical product database Reptox of the CSST (Quebec organization responsible for occupational safety and health), accessed April 25, 2009.
- Clément Gérome, Agnès Cadet-Taïrou, Michel Gandilhon, Maitena Milhet, Magali Martinez Et Thomas Néfau, « Psychoactive substances, users and markets: recent trends (2017-2018) », Trends , OFDT, French observatory of drugs and drug addictions, n O 129, ( read online ) .
- Lose S, CedrAgir/TREND, Recent trends and new drugs , December 2018 (OFDT, French observatory for drugs and drug addiction)
- Future , ‘ Definition | Absorption spectrometry – SAA | Futura Sciences » , on Future (consulted the ) .
- Randeniya, L. K., Vohralik, P. F. et plumb, I. C. (2002), Stratospheric ozone depletion at northern mid latitudes in the 21st century: The importance of future concentrations of greenhouse gases nitrous oxide and methane . Geophysical research letters, 29(4).
- (in) David R. Kanter , Claudia Wagner-Riddle , Peter M. Grayman et Eric A. Davidson , ‘ Improving the social cost of nitrous oxide » , Nature Climate Change , vol. 11, n O 12, , p. 1008–1010 (ISSN 1758-678X And 1758-6798 , DOI 10.1038/S41558-021-01226-Z , read online , consulted the ) .
- (in) Cotton F. A. et Wilkinson G. (1972), Advanced Inorganic Chemistry, a comprehensive text , 3 It is ed. , Interscience Publishers, John Wiley & Sons, p. 355 .
- Denis Richard, Jean-Louis Senon and Marc Valleur, Dictionary of drugs and dependencies , Paris, Larousse, , 626 p. (ISBN 2-03-505431-1 ) .
- D r Daniel Annequin, “A year after the Meopa out of the hospital reserve. Inventory ” [PDF] , Functional Pain Function Unit, Armand-Trousseau Children’s Hospital in Paris, National Center for Resources for Pain Combat (accessed October 28, 2018).
- “Annex 1: ARM CORRENCE DATE OF THE » [PDF] , National Center for Pain Combat Resource.
- “Annex 3: Afssaps recommendations concerning the Meopa ( ) » [PDF] , National Center for Pain Combat Resource.
- Group chaired by the D r Annequin, with the P r Corinne Lejus, D r Barbara Tourniiary, Elisabeth Fournier Charrière, Nada Sabourdin who coordinated the work subgroups; THE D r Nathalie Dumarcet of Afssaps ensuring general coordination. The Pediadol association ( pediadol.org ) has contributed to the logistical facilitation of these recommendations.
- ‘ http://www.afssaps.fr/var/afssaps_site/storage/Application/7b8bf3bf3bf0175D7C1EF72E65079AC4E56 E.PDF » ( Archive.org • Wikiwix • Archive.is • Google • What to do ?) , on AFSSAPS.FR .
- Caroline Victorri-Vigneau (2017), Clinical pharmacology – MEOPA use practices in a CHU: what compliance? ( MEOPA use practices in a university hospital: Which conformity? ), Therapies , vol. 72, n O 6, December 2017, p. 659-663 , DOI 10.1016/j.therap.2017.04.003 .
- ‘ Flowing up greenhouse gas concentrations: new record » , on OMM , (consulted the ) .
- (in) Ravishankara A.R, Daniel J.S et Portmann R.W (2009), Nitrous oxide (N 2 O ): the dominant ozone-depleting substance emitted in the 21st century [PDF] , Science , 326 (5949), 123-125.
- G. Tallec, Nitrous oxide emissions when treatment of nitrogen in treatment plant (Doctoral thesis), Marne-la-Vallée, enpc, , Parisian agglomeration.
- (in) P.J. Crutch and D.H. Bodily , ‘ Effects of nitrogen fertilizers and combustion on the stratospheric ozone layer » , Ambio , , p. 112-117 .
- (in) Roberto André Seriously, Rodrigo of the Silveira Nicoloso, Paule Celio Camio Luis Busi da Silva, Melissa Parola Mezzari, Celso Aita and Camila Rosan Wuadan, ‘ Determining the effects of tillage and nitrogen sources on soil N 2 O emission» , Soil and Tillage Research , n O 175, (DOI 10.1016/j.still.2017.08.011 , read online ) .
- (in) S. Perkins, ‘ Leafcutter ant ‘compost piles’ produce potent greenhouse gases » , .
- (in) R. L. Thompson , L. Lasaletta , P. K. Patra and C. Wilson , ‘ Acceleration of global N 2 O emissions seen from two decades of atmospheric inversion » , Nature Climate Change , vol. 9, n O 12, , p. 993–998 (ISSN 1758-6798 , DOI 10.1038/S41558-019-0613-7 , read online , consulted the ) .
- ‘ The upward trend continues: concentrations of greenhouse gas in the atmosphere have reached new heights in 2018 » , on World meteorological organization , (consulted the ) .
- (in) V. N. Armon , G. I. Panov , A. Uriarte and A. S. Noskov , ‘ Nitrous oxide in oxidation chemistry and catalysis application and production » , Elsevier , vol. 100, , p. 115-131 (DOI 10.1016/j.cattod.2004.12.012 ) .
- ‘ Overview of Greenhouse Gases – Nitrous Oxide » , US EPA (consulted the ) , Page 164 (document header listing).
- ‘ Nitrogen protoxide – n 2 O » , on citepa.org , (consulted the ) .
- ‘ Nitrogen oxide, a greenhouse gas that does not make anyone laugh » , on INRA.FR , (consulted the ) .
- (in) Kanter D., Mauzerall D.L., Ravishankara A.R., Daniel J.S., Portmann R.W., Grabiel P.M.,… et Galloway J.N. (2012), A post-Kyoto partner: considering the stratospheric ozone regime as a tool to manage nitrous oxide [PDF] , Proceedings of the National Academy of Sciences , 201222231
- Appendix 8.A [PDF] , Intergovernmental Panel on Climate Change Fifth Assessment Report , p. 731 .
- European Environment Agency and Agage , Trends in atmospheric concentrations of CO 2 , CH4 and N2O, March 20, 2019 (accessed July 7, 2019).
- Ravishankara A.R., Daniel J.S. ET Portmann R.W. (2009), Nitrous Oxide (N 2 O ): The Dominant Ozone-Depleting Substance Emitted in the 21st Century, Science , 326 (5949): 123–5, Bibcode : 2009Sci…326..123R, DOI 10.1126/science.1176985 , .
- Data , the European Environment Agency (accessed May 25, 2019)
- (in) Direct Global Warming Potentials p. 212
- Hebbles D.j. (2009), Nitrous oxide: no laughing matter , Science , 326, 5949, p. 56-57.
- (in) Portmann R.W, Daniel J.S. et ravishara a.r. (2012), Stratospheric ozone depletion due to nitrous oxide: influences of other gases , Phil. Trans. R. Soc. B , 367 (1593), 1256-1264.
- (in) Menon S., Denman K.L., Brasseur G., Chidthaisong A., Ciais P., Cox P. M.,… and Jacob D. (2007), Couplings between changes in the climate system and biogeochemistry ( n O LBNL-464E) , Lavance Bearkel lab. (Jlgar), Bethley, caved, calculations.
- (in) Crutzen P.J. (1970), The influence of nitrogen oxides on the atmospheric ozone content [PDF] , Quarterly Journal of the Royal Meteorological Society , 96 (408), 320-325.
- Chapuis, C., Guerquin, L. and Albaladejo, P. (2016), Are the drugs used in anesthesia really major pollutants? , The resuscitation anesthesia practitioner , 20 (4), 184-187
- WHO Model List of Essential Medicines, 18th list , April 2013.
- Yamakura T. et Harris R.A., « Effects of gaseous anaesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol », Anesthesiology , vol. 93, n O 4, , p. 1095–101 (PMID 11020766 , DOI 10.1097/00000542-20010000-00034 ) .
- Mennerick S., Jevtovic-Todorovic V., Todorovic S.M., Shen W., Olney J.W. et Zorumski C.F., « Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures », Journal of Neuroscience , vol. 18, n O 23, , p. 9716–26 (PMID 9822732 , DOI 10.1523/JNEUROSCI.18-23-09716.1998 ) .
- Gruss M., Bushell T.J., Bright D.P., Lieb W.R., Mathie A. et Franks N.P., « Two-pore-domain K + channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane », Molecular Pharmacology , vol. 65, n O 2, , p. 443–52 (PMID 14742687 , DOI 10.1124/mol.65.2.443 ) .
- Emmanouil D.E. et Quock R.M., ‘ Advances in Understanding the Actions of Nitrous Oxide », Anesthesia Progress , vol. 54, n O 1, , p. 9–18 (PMID 17352529 , PMCID 1821130 , DOI 10,2344/0003-3006 (2007) 54 [9: AIATAO] 2.0.CO; 2 ) .
- Roland M. Atkinson , J. Dewayne Green , Dennis E. Chenoweth et Judith Holmes Atkinson « Subjective Effects of Nitrous Oxide: Cognitive, Emotional, Perceptual and Transcendental Experiences », Journal of Psychedelic Drugs , vol. 11, n O 4, , p. 317–330 (DOI 10.1080/02791072.1979.10471415 ) .
- Diana J. Walker and James P. Noble « Within- and between-subject variability in the reinforcing and subjective effects of nitrous oxide in healthy volunteers », Drug and Alcohol Dependence , vol. sixty four, n O 1, , p. 85–96 (PMID 11470344 , DOI 10.1016/s0376-8716(00)00234-9 ) .
- Jay M., « Nitrous oxide: recreational use, regulation and harm reduction », Drugs and Alcohol Today , vol. 8, n O 3, , p. 22–25 (DOI 10,1108/17459265200800022 ) .
- S. Sakamoto , S. Nakao , M. Masuzawa ; Takefumi Without , Mervyn Maze , Nicholas P. Franks not koh Shingu « The differential effects of nitrous oxide and xenon on extracellular dopamine levels in the rat nucleus accumbens: a microdialysis study », Anesthesia and Analgesia , vol. 103, n O 6, , p. 1459–63 (PMID 17122223 , DOI 10.1213/01.ane.0000247792.03959.f1 , CiteSeer x 10.1.1.317.6613 ) .
- Benturquia N., Le Marec T., Scherrmann J.M. and noble F. ” Effects of nitrous oxide on dopamine release in the rat nucleus accumbens and expectation of reward », Neuroscience , vol. 155, n O 2, , p. 341–4 (PMID 18571333 , DOI 10.1016/j.neuroscience.2008.05.015 ) .
- LIGHTIGN Field F.J. it gillman m.a., « Role of dopamine mesolimbic system in opioid action of psychotropic analgesic nitrous oxide in alcohol and drug withdrawal », Clinical Neuropharmacology , vol. 19, n O 3, , p. 246–51 (PMID 8726543 , DOI 10.1097/00002826-199619030-00006 ) .
- Koyanagi S., Himukashi S., Mukaida K., SHICHINO T. ET FUKUDA K., «« Dopamine D2-like receptor in the nucleus accumbens is involved in the antinociceptive effect of nitrous oxide », Anesthesia and Analgesia , vol. 106, n O 6, , p. 1904–9 (PMID 18499630 , DOI 10.1213/ane.0B013E318172B15B , CiteSeer x 10.1.1.327.9838 ) .
- David H.N., Ansseau M., Lemaire M. and Abraini J.H., ” Nitrous oxide and xenon prevent amphetamine-induced carrier-mediated dopamine release in a memantine-like fashion and protect against behavioral sensitization », Biological Psychiatry , vol. 60, n O 1, , p. 49–57 (PMID 16427030 , DOI 10.1016/j.biopsych.2005.10.007 ) .
- N. Benturquia , S. The Guen , C. canestrelli , IN. Exchange , G. APIOU , B.P. Rocks and F. Noble « Specific blockade of morphine- and cocaine-induced reinforcing effects in conditioned place preference by nitrous oxide in mice », Neuroscience , vol. 149, n O 3, , p. 477–86 (PMID 17905521 , DOI 10.1016/j.neuroscience.2007.08.003 , read online ) .
- Ramsay D.S., Watson C.H., Leroux B.G., PRALL C.W. et kaiyala k.J., « Conditioned place aversion and self-administration of nitrous oxide in rats », Pharmacology Biochemistry and Behavior , vol. 74, n O 3, , p. 623–33 (PMID 12543228 , DOI 10.1016/S0091-3057 (02) 01048-1 ) .
- Wood R.W., Grubman J. et Weiss B., « Nitrous oxide self-administration by the squirrel monkey », The Journal of Pharmacology and Experimental Therapeutics , vol. 202, n O 3, , p. 491–9 (PMID 408480 ) .
- A good J.P. et galinkin J.L., « Psychotropic drugs used in anesthesia practice: abuse liability and epidemiology of abuse », Anesthesiology , vol. 90, n O 1, , p. 269–88 (PMID 9915336 , DOI 10.1097/00000542-19991000-00033 ) .
- Dohrn C.S., Liechtor J.L., Coalson D.W., Uitvlugt A., Deit H. Et Zacny J.P., « Reinforcing effects of extended inhalation of nitrous oxide in humans », Drug and Alcohol Dependence , vol. thirty first, n O 3, , p. 265–80 (PMID 8462415 , DOI 10.1016/0376-8716(93)90009-F ) .
- Walker D.J. Et good J.P., « Within- and between-subject variability in the reinforcing and subjective effects of nitrous oxide in healthy volunteers », Drug and Alcohol Dependence , vol. sixty four, n O 1, , p. 85–96 (PMID 11470344 , DOI 10.1016/S0376-8716(00)00234-9 ) .
- Emmanouil DE, Johnson CH, Quock RM, « Nitrous oxide anxiolytic effect in mice in the elevated plus maze: mediation by benzodiazepine receptors », Psychopharmacology , vol. 115, n you 1–2, , p. 167–72 (PMID 7862891 , DOI 10.1007/BF02244768 ) .
- Zacny JP, Yajnik S, Coalson D, Lichtor Jl, Apfelbaum JL, Rupani G, Young C, Thapar P, Klafta J, « Flumazenil may attenuate some subjective effects of nitrous oxide in humans: a preliminary report », Pharmacology Biochemistry and Behavior , vol. 51, n O 4, , p. 815–9 (PMID 7675863 , DOI 10.1016/0091-3057 (95) 00039-Y ) .
- Berkwitz ba, Finck ad, Hynes MD, Ngai S, « « Tolerance to nitrous oxide analgesia in rats and mice », Anesthesiology , vol. 51, n O 4, , p. 309–12 (PMID 484891 , DOI 10.1097/00000542-197910000-00006 ) .
- Branda Em, Ramza JT, Cahill FJ, Tseng LF, Quock RM, « Role of brain dynorphin in nitrous oxide antinociception in mice », Pharmacology Biochemistry and Behavior , vol. 65, n O 2, , p. 217–21 (PMID 10672972 , DOI 10.1016/S0091-3057 (99) 00202-6 ) .
- Guo Tz, Davies MF, Kingery WS, Patterson Aj, Limbird and, Maze M, « Nitrous oxide produces antinociceptive response via alpha2B and/or alpha2C adrenoceptor subtypes in mice », Anesthesiology , vol. 90, n O 2, , p. 470–6 (PMID 9952154 , DOI 10.1097/00000542-199902000-00022 ) .
- Sawamura S, Kingery WS, Davies MF, Agashe GS, Clark JD, Koblika BK, Hashimoto T et Maze M, « Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of [alpha] 2B adrenoceptors », J. Neurosci. , vol. 20, n O 24, , p. 9242–51 (PMID 11125002 , DOI 10.1523/JNEUROSCI.20-24-09242.2000 ) .
- Maze M. et FUJINAGA M., «« Recent advances in understanding the actions and toxicity of nitrous oxide », Anaesthesia , vol. 55, n O 4, , p. 311–4 (PMID 10781114 , DOI 10.1046/j.1365-2044.2000.01463.x ) .
- ‘ Nitrogen oxide (nitrous oxide) » , on Mecamotors .
- ‘ Fuels & Fluids Used By Germany: GM-1 » , on Air Force Resource Center .
- Claire Boutron, Monique Mathieu-Nolf, Nicolas Pety, Marc Deveaux, « Diverted uses of nitrogen oxide », Annals of analytical toxicology , vol. XII, n O 3, ( read online )
- Garbaz L, Mispelaere D, Boutemy M et Jenieaux V., Pneumothorax and voluntary nitrogen protoxide , Rev. Mal. Respir. , May 2007, 24 (5): 622-4.
- Lise Loumé (2015), “An 18 -year -old Briton dies after inhaling hilarious gas” , Science and future , July 29, 2015.
- Valentine Leroy, “Recreational drugs: nitrogen oxide no longer makes people laugh” , on Illicit-trade.com , October 22, 2019.
- Luc de Haro, Jocelyne Arditti and Catherine Messina-Gourlot, Comité de coordination of toxicovigilance, Deviation of use of nitrogen (report), Directorate General of Health, , twelfth p. ( read online [PDF] ) .
- Agathe Peplinski, « “Hilarious gas”: several cities are worried about nitrogen oxide », Le Figaro , ( read online ) .
- Cécile Bidault, ” “Hilarious gas”: serious cases linked to nitrogen oxide consumption are multiplying in the North », France Bleu Nord , ( read online ) .
- ‘ Drugs: Hilarious gas is wreaking havoc », L’Express , ( read online ) .
- Sarah Finger, « Montpellier: the amazing success of hilarious gas », Release , ( read online ) .
- Elsa Mari, « Nitrogen oxide alert, laughter drugs », The Parisian , ( read online ) .
- Senate, ‘ Dangerous uses of nitrogen oxide » .
- ‘ “Hilarious gas”: senators vote for the ban on sales to minors », West France , ( read online ) .
- ‘ He makes people hammer and polluted, Toulouse prohibits “hilarious gas” » , on 20minutes.fr (consulted the ) .
- Belgian, ‘ Almost 2 tonnes of nitrogen oxide cartridges collected by Brussels Cleanliness in 3 weeks » , on lavenir.net , (consulted the )
- Belgian, ‘ Nitrogen oxide cartridges and bottles damage the incinerators » , on RTBF , (consulted the )
- AIR, ‘ Explosion in the oven of a non -hazardous waste incinerator » , on ARIA, the reference to feedback on technological accidents , (consulted the )
- BFM Business, ‘ When the hilarious gas explodes in waste incineration factories » , on BFM Business , (consulted the )
- European Parliament and Council of Europe, ” Directive 95/2/EC concerning food additives other than dyes and sweeteners », Official newspaper , n O L 61, ( résumé , read online ) .
- Cutayar J and Pean J.L (2007), Use of nitrogen oxide (n 2 O ) ou d’un mélange de gaz comportant du protoxyde d’azote comme agent édulcorant de produits agroalimentaires, World Patent Wo/2007/090939
- Robert R. Luwerys, Vincent Hufroid et Perrine, Industrial toxicology and professional poisoning ( extract )
- Nitrous oxide , brochure published and distributed by the Spiritek association in partnership with the cities of Lille and Tourcoing, the Lille-Métropole Urban Community and the General Council of the North Department.
- “Nitrogen oxide, a hilarious gas that does not make doctors laugh at all” , on lemonde.fr , December 28, 2018 (accessed April 25, 2019)
- (in) Michel Hautefeuille and Dan Véléa, Synthetic drugs , Presses Universitaires de France, coll. “What do I know? “( n O 3625), , 127 p. (ISBN 978-2-13-052059-7 , OCLC 300468465 ) .
- Jordan J.T., Weiser J. et Van Ness P.C., Unrecognized cobalamin deficiency, nitrous oxide, and reversible subacute combined degeneration , Neurol. Clin. Pract. , August 2014, 4 (4): 358-361, DOI 10.1212/CPJ.000000000000001 .
- Chaugny C., Simon J., Collin-Masson H., De Beauchêne M., Cabral D., Fagniez O. et Veyssier-Belot C., Deficiency vitamine B twelfth by toxicity of nitrogen oxide: an unknown cause of combined sclerosis of the marrow , Rev. With. Internal , May 2014, 35 (5): 328-32, DOI 10.1016/j.revmed.2013.04.018 .
- (in) Michael A. Miller, Vicky Martinez, Richard McCarthy et Manish M. Patel, « Nitrous oxide whippit abuse presenting as clinical B twelfth deficiency and ataxia », American Journal of Emergency Medicine , vol. 22, n O 2, mars 2004 ( read online ).
- Deacon R., Lumb M., Perry J. et al. , Inactivation of methionine synthase by nitrous oxide , EUR. J. BIOCHEM , 1980, 104: 419–423
- Kwon YJ, Rho JH, Hwang J et Baek SH, Unhappy End of « Happy Balloons »: Subacute Combined Degeneration Caused by Nitrous Oxide Gas , J. Clin. Neurol. , October 26, 2018.
- Egan W, Steinberg E et Rose J, Vitamin B twelfth deficiency-induced neuropathy secondary to prolonged recreational use of nitrous oxide , Am. J. Emerg. With. , September 2018, 36 (9): 1717.e1-1717.e2, DOI 10.1016/J.AJA.2018.05.029 ( résumé )
- Antonucci mu, Subacute Combined Degeneration from Recreational Nitrous Oxide Inhalation , J. Emerg. With. From 2018, 54 (5): e105-e107, DOI 10.1016/j.jemermed.2018.01.045 .
- Keddie s, Adams A, Kelso arc, Turner B, Schmierer K, GNANAPAVAN S, PLINDS, GIOVANNI G, BASNETT I et Noyce AJ, No laughing matter: subacute degeneration of the spinal cord due to nitrous oxide inhalation , J. Neurol. , May 2018, 265 (5): 1089-1095, DOI 10.1007/s00415-018-8801-3 .
- Buizert A, Sharma r et heads h, When the Laughing Stops: Subacute Combined Spinal Cord Degeneration Caused by Laughing Gas Use , J. Addict. Med. , May-June 2017, 11 (3): 235-236, DOI 10.1097/ADM.0000000000000295 ( résumé )
- Johnson K, Mikhail P, Kim MG, Bosco A et Huynh W, Recreational nitrous oxide-associated neurotoxicity , J. Neurol. Neurosurg. Psychiatry , August 2018, 89 (8): 897-898, DOI 10.1136 / JNNP-2017-317768 ( résumé )
- Chaugny, C., Simon, J., Collin-Masson, H., De Beauchêne, M., Cabral, D., Fagniez, O. et Veyssier-Belot, C. (2014), Vitamin B12 deficiency by toxicity of nitrogen oxide: an unknown cause of combined sclerosis of the marrow . The review of internal medicine, 35 (5), 328-332.
- Morris n, Lynch K et Greenberg Sa (2015), Severe motor neuropathy or neuronopathy due to nitrous oxide toxicity after correction of vitamin B twelfth deficiency , Muscle Nerve , April, 51 (4): 614-6, DOI 10.1002 / to.24482 .
Bibliography [ modifier | Modifier and code ]
- Saito, R., Patra, P.K., Deutscher, N., Wunch, D., Ishijima, K., Sherlock, V., Blumennck, T., Dohe, S., Griffith, D., Hase, F., heikkinen , P., Kyrö, E., Macatayan, R., Mendonca, J., Messserschmidt, J., Morino, I., Notholt, J., Rettinger, M., Strong, K., Sussmann, R. , T. (2012), Technical Note: Latitude-time variations of atmospheric column-average dry air mole fractions of CO 2 , CH4 and N2O, Atmos. Chem. Phys. , 12, 7767-7777, DOI 10.5194/acp-12-7767-2012 .
- Schilt A. et al. (2010), Atmospheric nitrous oxide during the last 140,000 years , Earth Planet. Sci. Lett. , 300, 33–43.
Related articles [ modifier | Modifier and code ]
external links [ modifier | Modifier and code ]








Recent Comments