Norbornene — Wikipedia
A wikipedia article, free l’encyclopéi.
| Norbornène | |
  |
|
| Identification | |
|---|---|
| DCI | norbornène |
| UICPA name | Bicyclo [2.2.1] HEPT-2-ENE |
| Synonyms |
norbornyne |
| N O CAS | |
| N O Echa | 100.007.152 |
| N O THIS | 207-866-0 |
| N O RTECS | RB7900000 |
| Pubchem | 10352 |
| SMILES | |
| Inches |
|
| Appearance | Solid white, tangy smell [ first ] |
| Chemicals | |
| Formula | C 7 H ten [Isomers] |
| Molar mass [ 2 ] | 94,154 3 ± 0.006 3 g/mol C 89,3 %, H 10,71 %, |
| Physical properties | |
| T° fusion | 44 To forty six °C [ 3 ] |
| T° boiling | 96 °C [ 3 ] |
| Solubility | 130 mg · l -first (water, 25 °C ) [ 4 ] |
| Flash point | −14 °C (closed cup) [ 3 ] |
| Saturating steam pressure | 32.6 hPa ( 65 °C ) [ first ] |
| Precautions | |
| SGH [ 3 ] , [ first ] | |
  H228 , H411 , P210 And P262 H228 : Flammable inflammable material |
|
| NFPA 704 [ 3 ] | |
|
|
|
| Transport [ 3 ] | |
|
|
|
| Ecotoxicology | |
| DL 50 | > 5 ml/kg (rabbit, dermal) [ 5 ]
11,3 g/kg (rat, oral) [ 5 ] |
| LogP | 3.24 [ first ] |
|
|
|
| IS units and CNTP , unless otherwise stated. | |
| modifier |
|
The norbornène , norbornyne or norcampène is a hydrocarbon punctuated by Formula C 7 H ten . It is a solid white with a spicy sour smell.
The molecule consists of a cycle of cyclohexene with a methylene bridge between the carbon C-3 and C-6. Because of its double bond, it undergoes a certain cyclic constraint and has a significant reactivity.
Norbornian has a structure similar to other bicyclic compounds, the Norbornadiene which has the same structure with an additional double connection, and the Norbornane without any double connection.
Norbornian, like many of its derivatives, is synthesized by Diels-alder reaction between cyclopentadiene and ethylene [ 6 ] , [ 7 ] .
The Norbornian undergoes hydration in an acidic environment with water to form Norborneol (in) .
Norbornène does not have many practical applications. It is used to synthesize intermediaries of pharmaceutical compounds, pesticides, certain special perfumes and in organic synthesis in general. Combined with ethylene, the Norbornian reacts by forming a copolymer of Cyclic Oléfine.
Polynorbornènes [ modifier | Modifier and code ]
The Norbennes are important monomers in polymerizations by opening of cycle by metathesis (broken) with for example the catalyst of Grubbs.
THE polynorbornènes are polymers with high glass transition temperatures and large optical transparency.
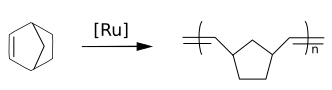
In addition to the breaks, the Norbornian can undergo a polymerization by addition-vinyl.
- ‘ Norbornène fiche » , Merck [PDF] (consulted the ) .
- Molar mass calculated after ‘ Atomic weights of the elements 2007 » , on www.chem.qmul.ac.uk .
- Compound sigma-aldrich sheet Bicyclo[2.2.1 HEPT-2’s] , accessed September 29, 2012.
- (in) ‘ Norbornène » , on Chedplus , accessed September 29, 2012
- American Industrial Hygiene Association Journal , vol. 30, p. 470, 1969, PubMed
- Paul Binger, Petra Wedemann et Udo H. Brinker, Cyclopropene: A New Simple Synthesis and its Diels-Alder Reaction with Cyclopentadiene , Org. Synth., coll. « vol. 10 », p. 231
- Masaji ODA, TAKESHI KAWASE, TOMOAKI OKADA et TETSUYA ENOMOTO, 2-Cyclohexene-1,4-dione , Org. Synth., coll. « vol. 9 », p. 186


Recent Comments