Topiramate — Wikipedia
A wikipedia article, free l’encyclopéi.
| Topiramate | |
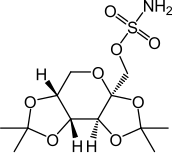 |
|
| Identification | |
|---|---|
| UICPA name | Sulfamate de ((3AS, 5AR, 8AR, 8BS) -2,2,7-7-Tétméthyltétrahydro-3AH-Bis [1,3] Dioxolo [4,5-b: 4 ‘, 5’-D] pyran-3a -Yl] Méthyle |
| Synonyms |
Sulifamate 2, 4,5-besydy (1-Maryism)-Malimy-aretante). |
| N O CAS | |
| N O Echa | 100.129.713 |
| Code ATC | N03 |
| DrugBank | AprD00237 |
| Pubchem | 5284627 |
| SMILES |
|
| Inches |
|
| Chemicals | |
| Formula | C twelfth H 21 NO 8 S [Isomers] |
| Molar mass [ first ] | 339,362 ± 0.019 G/MOL C 42,47 %, H 6,24 %, N 4,13 %, O 37,72 %, S 9,45 %, |
| Pharmacokinetic data | |
| Bio -supplood | 80% |
| Metabolism | 30% liver, 70% is excreted unchanged |
| Half-life of élim. | 19 to 11 p.m. |
| Excretion |
70% renal (the form unchanged) |
| Therapeutic considerations | |
| Administration | oral |
|
|
|
| IS units and CNTP , unless otherwise stated. | |
| modifier |
|

The topiramate is an antiepileptic drug used for generalized or partial type epilepsies. It is found, in France, under the name of Epitomax and in the United States, Canada, the United Kingdom, Australia and certain other countries under the name of Topamax.
It can also be used as a preventive treatment for migraine attacks.
Studies have also been carried out on its usefulness in the treatment of the effects induced by ketamine, then used as a model of schizophrenia.
Il Casiit a Durrivé du Frucstose [ 2 ] .
The bioavailability is more than 80%, without significant modification by food intake. Its half-life is around 24 hours and the excretion is renal. Phenytoin and carbamazepine halved its half-life, imposing an increase in doses [ 3 ] .
- Epilepsy
Epilepsy of the child and adolescent. This is its first use. In children, it is indicated for the treatment of Lennox-Gastaut syndrome [Ref. necessary] . - Migraines
Furthermore, it significantly decreases the number of migraine crises when given in basic treatment [ 4 ] . - Borderline personality disorder
Borderline personality disorder including mood instability. This indication is recommended by a review of the 2010 Cochrane collaboration [ 5 ] . - Bipolar disorder
However, this indication is criticized by a Cochrane review which concludes in insufficient efficiency [ 6 ] .
Studies have also been carried out on its usefulness in treating the effects brought about by ketamine, then used as a model of schizophrenia [ 7 ] , [ 8 ] .
The most frequent are the tingling sensations (paraesthesia present in half of the cases and leading to stopping treatment in just less than one in 10) and a fairly significant loss of weight during treatment [ 9 ] .
The ANSM warns against the teratogenicity of the topiramate: pregnant women are exposed to a risk 3 times greater of fetus malformations, including Bacs-de-de-de-livre or hypospadias. In addition, cases of neurodevelopmental disorders are reported [ ten ] . The study Jama Neurology of May 31, 2022 brings new elements. The ANSM then modifies the prescription conditions mentioning that the observed risk of major malformations amounts to 8.2%, as well as the risk of the occurrence of autistic spectrum disorders which is between 2 and 6%, finally a risk of an intellectual impairment which located between 1 and 8%.
- Molar mass calculated after ‘ Atomic weights of the elements 2007 » , on www.chem.qmul.ac.uk .
- (in) Shank RP, Maryanoff BE, « Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate » CNS Neurosci Ther . 2008; 14: 120-42.
- (in) Bialer M, Doose DR, Murthy B. et al. « Pharmacokinetic interactions of topiramate » Clin Pharmacokinet . 2004; 43: 763-80.
- (in) Brandes JL, Saper JR, Diamond M. et al. « Topiramate for migraine prevention: a randomized controlled trial » PEOPLE . 2004; 291: 965-73.
- (in) Klaus body , Birgit Völlm , Happen Back , Antje Timber And Jutta M Drugs , ‘ Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials » , British Journal of Psychiatry , vol. 196, n O 1, , p. 4–12 (DOI 10.1192/bjp.bp.108.062984)
- (in) Fireplaces Vasudev , Karine MacRitchie , John Geddes , Stuart Watson , Allan H Young and allan h Young , ‘ Topiramate for acute affective episodes in bipolar disorder » , Cochrane database of systematic reviews (Online) , n O 1, , p. CD003384 (PMID 16437453, DOI 10.1002/14651858.CD003384.pub2)
- (in) Deutsch Si, Rosse Rb, Billingslea EN, Bellack MP, Mastropaolo J, ‘ Topiramate antagonizes MK-801 in an animal model of schizophrenia » , EUR J PHARMACOL , vol. 449, n you 1-2, , p. 121-5. (PMID 12163115 )
- (in) Micallef J, Gavadan G, Burle B, Blin O, Hasbroucq T, ‘ A study of a topiramate pre-treatment on the effects induced by a subanaesthetic dose of ketamine on human reaction time » , Neurosci Lett , vol. 369, n O 2, , p. 99-103. (PMID 15450676 )
- (in) Edvinsson L, Linde M, « New drugs in migraine treatment and prophylaxis: telcagepant and topiramate » Lancet . 2010; 376: 645-55.
- ANSM – Antiepileptics during pregnancy: current state of knowledge on the risks of malformations and neur -developmental disorders – Information point
Recent Comments