Criminal Acido – Wikipedia
from Wikipedia, L’Encilopedia Libera.
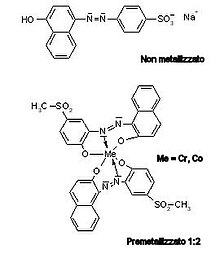
I acid dyes , O Anionic dyes , they are a class of dyes consisting of sodium salts of aromatic sulfonic acids that are applied in acid solutions. They are divided, depending on the presence or absence of a metal ion within the coloring molecule, in “non -metallic” and “pre -ultrasound”. The latter can be 1: 1 or 1: 2, depending on the ratio between the metal ion and a coloring molecule. The molecular structure, generally small and short, is characterized by multiple anonymous groups and chromogenic parts belonging to the classes of the azo (mono or bis, 65%), anthraquinone (for dark tones, 15%) and the trifenylmethane (12% ). Auxocrympt groups can also be present (-Oh or -Nh 2 ) and hydrophobic parts (long chain alkilical groups) to increase solidity.
Given the presence of sulfonic groups, they are soluble dyes in water whose solubility increases with the temperature.
Antrachinonic are more solid to light and damp treatments.
The trifenylmetatics have brighter tones, but they are the least solid in light.
Among the pre -trammelon are distinguished:
- 1: 1 – They are used only in a strongly acidic environment and only with wool
- 1: 2 – They are used for wool, silk and polyamid fiber. They can be both soluble and insoluble; 1: 2 solubles require a weakly acidic environment, while the insoluble ones are applied as dispersed dyes.
Depending on the fiber, the pH to which the dyeing takes place and from the speed of tincture requested, the acid dyes are divided into dyeing subclasses.
Class I [ change | Modifica Wikitesto ]
They tinge protein fibers with pH values between 2 and 4 (you use H 2 SO 4 or Hcooh). The acid dyes of this subclasse are characterized by a good equalization, even if they need 2 SO 4 (or spare soap for the tincture of the silk) as equal to delay the penetration of the dye, competing with it. They give poor solidity.
Class II [ change | Modifica Wikitesto ]
The dyes of this subclasse, called “follone”, tinge protein fibers with pH values between 4 and 6 (you use ch 3 Cooh and little na 2 SO 4 ). The acid dyes of this subclasse are characterized by an equalization and intermediate solidity. They need sodium sulphate, but its egarling action is limited to the aggregation of the dye.
The dyes of this subclasse, called “superfolone”, tinge protein fibers with pH values between 6 and 8 (you use that 3 Coona). The acid dyes of this subclasse are characterized by bad equalization, and consequently need auxiliary products to encourage egalization. They give good solidity and are milling dyes (i.e. excellent for crowd because very solid).
Group a [ change | Modifica Wikitesto ]
They dye the Polyamid fibers with pH between 4.5 and 5.5 and are indicated for clear and medium tones. They have a good equalization but limited humid solidity.
Group B [ change | Modifica Wikitesto ]
They dye the Polyamid fibers with pH between 6 and 7 and are indicated for intense tones. They have an average equalization and excellent wet solidity. Since they are characterized by a faster absorption than the coloring of the group A requires the use of an egalizing that may be a cationic surfactant or an anionic small chain auonic surfactant.
Both for the group A and for group B it is possible to make post-transtuments to improve the fixation of the dye with natural tannins (for dark tones) or with cationic polyerials of synthesis (for clear tones).
The non -metallic mainly form ionic links between the anionic groups of the coloring molecule and the cationic groups on the fiber:
Col-SO 3 – + NH 3 -L-COOH
For this reason, the wool is tinged to a pH that increases the positive charge of the fiber to the maximum.
In addition to the ionic link between the anionic group of the molecule and the cationic groups of fiber, the Ionian bonds form bonds between metallic (generally chrome) and anonymous group groups:
Col-Cr + – OOC-L-NH 2
and between metal ion and free electronic doubles present on the nitrogen atoms of the fiber. The 1: 2, having two metal ions for coloring molecule available, form a greater number of ties, showing more solidity for this.
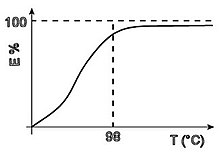
The tincture takes place in the watery bathroom. The pH value depends on the subclass to which the dye belongs, and must always be well controlled. The temperature has the effect of increasing the tincture speed; For subclass III the increase must be slower to improve equalization.
The tincture with acid dyes of the polyamidic fibers requires that the latter have a large number of terminal amino groups.
- Franco Corbani, Nobilization of textiles – Volume II , Cotton Textile Center and Clothing.
Recent Comments