Chemical compound
Telapristone
Other names
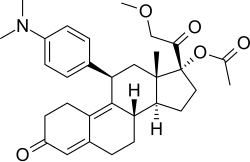
CDB-4124; Proellex; Progenta; 17β-(Acetyloxy)-11β-[4-(dimethylamino)phenyl]-17α-(2-methoxyacetyl)estra-4,9-dien-3-one
Drug class
Selective progesterone receptor modulator
Legal status
[(8S ,11R ,13S ,14S ,17R )-11-[4-(Dimethylamino)phenyl]-17-(2-methoxyacetyl)-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a ]phenanthren-17-yl] acetate
CAS Number
PubChem CID
PubChem SID
DrugBank
ChemSpider
UNII
ChEMBL
CompTox Dashboard (EPA )
Formula
C 31 H 39 N O 5
Molar mass
−1
3D model (JSmol)
O=C5C=C3/C(=C2/[C@@H](c1ccc(N(C)C)cc1)C[C@@]4([C@@](OC(=O)C)(C(=O)COC)CC[C@H]4[C@@H]2CC3)C)CC5
InChI=1S/C31H39NO5/c1-19(33)37-31(28(35)18-36-5)15-14-27-25-12-8-21-16-23(34)11-13-24(21)29(25)26(17-30(27,31)2)20-6-9-22(10-7-20)32(3)4/h6-7,9-10,16,25-27H,8,11-15,17-18H2,1-5H3/t25-,26+,27-,30-,31-/m0/s1
Y
Key:JVBGZFRPTRKSBB-MJBQOYBXSA-N
Y
(verify)
Telapristone (INN ), as telapristone acetate (proposed brand names Proellex , Progenta ; former code name CDB-4124 ), is a synthetic, steroidal selective progesterone receptor modulator (SPRM) related to mifepristone which is under development by Repros Therapeutics for the treatment of breast cancer, endometriosis, and uterine fibroids.[1] [2] It was originally developed by the National Institutes of Health (NIH), and, as of 2017, is in phase II clinical trials for the aforementioned indications.[1] In addition to its activity as an SPRM, the drug also has some antiglucocorticoid activity.[3]
See also [ edit] References [ edit] External links [ edit]
PR
Agonists
Testosterone derivatives: Progestins: 6,6-Difluoronorethisterone6,6-Difluoronorethisterone acetate
17α-Allyl-19-nortestosterone
Allylestrenol
Altrenogest
Chloroethynylnorgestrel
Cingestol
Danazol
Desogestrel
Dienogest
Ethinylandrostenediol
Ethisterone
Ethynerone
Etonogestrel
Etynodiol
Etynodiol diacetate
Gestodene
Gestrinone
Levonorgestrel
Levonorgestrel esters (e.g., levonorgestrel butanoate)
Lynestrenol
Lynestrenol phenylpropionate
Metynodiol
Metynodiol diacetate
Norelgestromin
Norethisterone (norethindrone)
Norethisterone esters (e.g., norethisterone acetate, norethisterone enanthate)
Noretynodrel
Norgesterone
Norgestimate
Norgestrel
Norgestrienone
Norvinisterone
Oxendolone
Quingestanol
Quingestanol acetate
Tibolone
Tigestol
Tosagestin; Anabolic–androgenic steroids: 11β-Methyl-19-nortestosterone
11β-Methyl-19-nortestosterone dodecylcarbonate
19-Nor-5-androstenediol
19-Nor-5-androstenedione
19-Nordehydroepiandrosterone
Bolandiol
Bolandiol dipropionate
Bolandione
Dimethisterone
Dienedione
Dienolone
Dimethandrolone
Dimethandrolone buciclate
Dimethandrolone dodecylcarbonate
Dimethandrolone undecanoate
Dimethyldienolone
Dimethyltrienolone
Ethyldienolone
Ethylestrenol (ethylnandrol)
Methyldienolone
Metribolone (R-1881)
Methoxydienone (methoxygonadiene)
Mibolerone
Nandrolone
Nandrolone esters (e.g., nandrolone decanoate, nandrolone phenylpropionate)
Norethandrolone
Normethandrone (methylestrenolone, normethandrolone, normethisterone)
RU-2309
Tetrahydrogestrinone
Trenbolone (trienolone)
Trenbolone esters (e.g., trenbolone acetate, trenbolone enanthate)
Trendione
Trestolone
Trestolone acetate
MixedSPRMs )
Antagonists
mPR PAQR )
Recent Comments